Inflammation and cardiac dysfunction during sepsis, muscular dystrophy, and myocarditis
- PMID: 27574633
- PMCID: PMC4978107
- DOI: 10.4103/2321-3868.123072
Inflammation and cardiac dysfunction during sepsis, muscular dystrophy, and myocarditis
Abstract
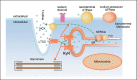
Inflammation plays an important role in cardiac dysfunction under different situations. Acute systemic inflammation occurring in patients with severe burns, trauma, and inflammatory diseases causes cardiac dysfunction, which is one of the leading causes of mortality in these patients. Acute sepsis decreases cardiac contractility and impairs myocardial compliance. Chronic inflammation such as that occurring in Duchenne muscular dystropshy and myocarditis may cause adverse cardiac remodeling including myocyte hypertrophy and death, fibrosis, and altered myocyte function. However, the underlying cellular and molecular mechanisms for inflammatory cardiomyopathy are still controversial probably due to multiple factors involved. Potential mechanisms include the change in circulating blood volume; a direct inhibition of myocyte contractility by cytokines (tumor necrosis factor (TNF)-α, interleukin (IL)-1β); abnormal nitric oxide and reactive oxygen species (ROS) signaling; mitochondrial dysfunction; abnormal excitation-contraction coupling; and reduced calcium sensitivity at the myofibrillar level and blunted β-adrenergic signaling. This review will summarize recent advances in diagnostic technology, mechanisms, and potential therapeutic strategies for inflammation-induced cardiac dysfunction.
Keywords: Burn; Duchenne muscular dystrophy; cardiac dysfunction; contractility; inflammation; sepsis.
Figures
References
-
- McEvoy C, Kollef MH. Determinants of hospital mortality among patients with sepsis or septic shock receiving appropriate antibiotic treatment. Curr Infect Dis Rep. 2013;15:400–6. - PubMed
-
- Xie B, Xiao SC, Peng XD, Zhu SH, Lv KT, Li HY, et al. Epidemiology and outcome analysis of severe extensive burns: A 12-year summary of 103 cases in a burn center in China. J Burn Care Res. 2012;33:e127–32. - PubMed
-
- Avlas O, Fallach R, Shainberg A, Porat E, Hochhauser E. Toll-like receptor 4 stimulation initiates an inflammatory response that decreases cardiomyocyte contractility. Antioxid Redox Signal. 2011;15:1895–909. - PubMed
-
- Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1 alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–53. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

