(18)F-FDG PET/CT for Monitoring the Response of Breast Cancer to miR-143-Based Therapeutics by Targeting Tumor Glycolysis
- PMID: 27574783
- PMCID: PMC5023410
- DOI: 10.1038/mtna.2016.72
(18)F-FDG PET/CT for Monitoring the Response of Breast Cancer to miR-143-Based Therapeutics by Targeting Tumor Glycolysis
Abstract
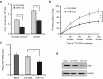
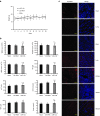
Increased glucose utilization is a hallmark of cancer, and tumor metabolism is emerging as anticancer target for therapeutic intervention. Triple-negative breast cancers TNBC are highly glycolytic and show poor clinical outcomes. We previously identified hexokinase 2, the major glycolytic enzyme, as a target gene of miR-143 in TNBC. Here, we developed a therapeutic formulation using cholesterol-modified miR-143 agomir encapsulated in a neutral lipid-based delivery agent that blocked tumor growth and glucose metabolism in TNBC tumor-bearing mice when administered systemically. The antioncogenic effects were accompanied by a reduction in the direct target hexokinase 2 and [(18)F]-fluorodeoxyglucose ((18)F-FDG) uptake based on positron emission tomography/computed tomography. Treatment with miR-143 formulation has minimal toxic effects and mice tolerated it well. Thus, we demonstrated that miR-143 is a robust inhibitor of the Warburg effect and an effective therapeutic target for TNBC. In addition, (18)F-FDG positron emission tomography/computed tomography can be used to specifically monitor the response of TNBC to miR-143-based therapeutics by targeting tumor glycolysis.
Figures



References
-
- Torre, LA, Bray, F, Siegel, RL, Ferlay, J, Lortet-Tieulent, J and Jemal, A (2015). Global cancer statistics, 2012. CA Cancer J Clin 65: 87–108. - PubMed
-
- Carey, L, Winer, E, Viale, G, Cameron, D and Gianni, L (2010). Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 7: 683–692. - PubMed
-
- Kassam, F, Enright, K, Dent, R, Dranitsaris, G, Myers, J, Flynn, C et al. (2009). Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 9: 29–33. - PubMed
-
- Hanahan, D and Weinberg, RA (2011). Hallmarks of cancer: the next generation. Cell 144: 646–674. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

