RhoH participates in a multi-protein complex with the zinc finger protein kaiso that regulates both cytoskeletal structures and chemokine-induced T cells
- PMID: 27574848
- PMCID: PMC5927543
- DOI: 10.1080/21541248.2016.1220780
RhoH participates in a multi-protein complex with the zinc finger protein kaiso that regulates both cytoskeletal structures and chemokine-induced T cells
Abstract
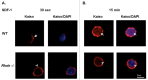
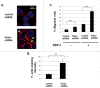
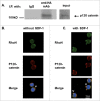
RhoH is a haematopoietic -specific, GTPase-deficient Rho GTPase that plays an essential role in T lymphocyte development and haematopoietic cell migration. RhoH is known to interact with ZAP70 in T cell receptor (TCR) signaling and antagonize Rac GTPase activity. To further elucidate the molecular mechanisms of RhoH in T cell function, we carried out in vivo biotinylation and mass spectrometry analysis to identify new RhoH-interacting proteins in Jurkat T cells. We indentified Kaiso by streptavidin capture and confirmed the interaction with RhoH by co-immunoprecipitation. Kaiso is a 95 kDa dual-specific Broad complex, Trantrak, Bric-a-brac/Pox virus, Zinc finger (POZ-ZF) transcription factor that has been shown to regulate both gene expression and p120 catenin-associated cell-cell adhesions. We further showed that RhoH, Kaiso and p120 catenin all co-localize at chemokine-induced actin-containing cell protrusion sites. Using RhoH knockdown we demonstrated that Kaiso localization depends on RhoH function. Similar to the effect of RhoH deficiency, Kaiso down-regulation led to altered cell migration and actin-polymerization in chemokine stimulated Jurkat cells. Interestingly, RhoH and Kaiso also co-localized to the nucleus in a time-dependent fashion after chemokine stimulation and with T cell receptor activation where RhoH is required for Kaiso localization. Based on these results and previous studies, we propose that extracellular microenvironment signals regulate RhoH and Kaiso to modulate actin-cytoskeleton structure and transcriptional activity during T cell migration.
Keywords: Kaiso; RhoH; actin-cytoskeleton; chemokine; migration.
Figures




References
-
- Dallery E, Galiegue-Zouitina S, Collyn-d'Hooghe M, Quief S, Denis C, Hildebrand MP, Lantoine D, Deweindt C, Tilly H, Bastard C, et al.. TTF, a gene encoding a novel small G protein, fuses to the lymphoma- associated LAZ3 gene by t(3;4) chromosomal translocation. Oncogene 1995; 10:2171-8; PMID:7784061 - PubMed
-
- Preudhomme C, Roumier C, Hildebrand MP, Dallery-Prudhomme E, Lantoine D, Lai JL, Daudignon A, Adenis C, Bauters F, Fenaux P, et al.. Nonrandom 4p13 rearrangements of the RhoH/TTF gene, encoding a GTP- binding protein, in non-Hodgkin's lymphoma and multiple myeloma. Oncogene 2000; 19:2023-32; PMID:10803463; https://doi.org/10.1038/sj.onc.1203521 - DOI - PubMed
-
- Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R, Dalla-Favera R. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 2001; 412:341-6; PMID:11460166; https://doi.org/10.1038/35085588 - DOI - PubMed
-
- Crequer A, Troeger A, Patin E, Ma CS, Picard C, Pedergnana V, Fieschi C, Lim A, Abhyankar A, Gineau L, et al.. Human RHOH deficiency causes T cell defects and susceptibility to EV-HPV infections. J Clin Invest 2012; 122:3239-47; PMID:22850876; https://doi.org/10.1172/JCI62949 - DOI - PMC - PubMed
-
- Sanchez-Aguilera A, Rattmann I, Drew DZ, Muller LU, Summey V, Lucas DM, Byrd JC, Croce CM, Gu Y, Cancelas JA, et al.. Involvement of RhoH GTPase in the development of B-cell chronic lymphocytic leukemia. Leukemia 2010; 24:97-104; PMID:19847197; https://doi.org/10.1038/leu.2009.217 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
