Pancreatic Cancer Surgical Resection Margins: Molecular Assessment by Mass Spectrometry Imaging
- PMID: 27575375
- PMCID: PMC5019340
- DOI: 10.1371/journal.pmed.1002108
Pancreatic Cancer Surgical Resection Margins: Molecular Assessment by Mass Spectrometry Imaging
Abstract
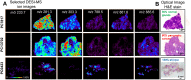
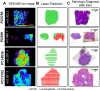
Background: Surgical resection with microscopically negative margins remains the main curative option for pancreatic cancer; however, in practice intraoperative delineation of resection margins is challenging. Ambient mass spectrometry imaging has emerged as a powerful technique for chemical imaging and real-time diagnosis of tissue samples. We applied an approach combining desorption electrospray ionization mass spectrometry imaging (DESI-MSI) with the least absolute shrinkage and selection operator (Lasso) statistical method to diagnose pancreatic tissue sections and prospectively evaluate surgical resection margins from pancreatic cancer surgery.
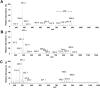
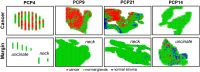
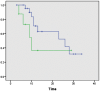
Methods and findings: Our methodology was developed and tested using 63 banked pancreatic cancer samples and 65 samples (tumor and specimen margins) collected prospectively during 32 pancreatectomies from February 27, 2013, to January 16, 2015. In total, mass spectra for 254,235 individual pixels were evaluated. When cross-validation was employed in the training set of samples, 98.1% agreement with histopathology was obtained. Using an independent set of samples, 98.6% agreement was achieved. We used a statistical approach to evaluate 177,727 mass spectra from samples with complex, mixed histology, achieving an agreement of 81%. The developed method showed agreement with frozen section evaluation of specimen margins in 24 of 32 surgical cases prospectively evaluated. In the remaining eight patients, margins were found to be positive by DESI-MSI/Lasso, but negative by frozen section analysis. The median overall survival after resection was only 10 mo for these eight patients as opposed to 26 mo for patients with negative margins by both techniques. This observation suggests that our method (as opposed to the standard method to date) was able to detect tumor involvement at the margin in patients who developed early recurrence. Nonetheless, a larger cohort of samples is needed to validate the findings described in this study. Careful evaluation of the long-term benefits to patients of the use of DESI-MSI for surgical margin evaluation is also needed to determine its value in clinical practice.
Conclusions: Our findings provide evidence that the molecular information obtained by DESI-MSI/Lasso from pancreatic tissue samples has the potential to transform the evaluation of surgical specimens. With further development, we believe the described methodology could be routinely used for intraoperative surgical margin assessment of pancreatic cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2436-41. doi: 10.1073/pnas.1400274111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24550265 Free PMC article.
-
In situ DESI-MSI lipidomic profiles of mucosal margin of oral squamous cell carcinoma.EBioMedicine. 2021 Aug;70:103529. doi: 10.1016/j.ebiom.2021.103529. Epub 2021 Aug 12. EBioMedicine. 2021. PMID: 34391097 Free PMC article.
-
Identification of diagnostic metabolic signatures in clear cell renal cell carcinoma using mass spectrometry imaging.Int J Cancer. 2020 Jul 1;147(1):256-265. doi: 10.1002/ijc.32843. Epub 2020 Jan 21. Int J Cancer. 2020. PMID: 31863456 Free PMC article.
-
Desorption electrospray ionization mass spectrometry imaging (DESI-MSI) in disease diagnosis: an overview.Anal Methods. 2023 Aug 10;15(31):3768-3784. doi: 10.1039/d3ay00867c. Anal Methods. 2023. PMID: 37503728 Review.
-
Ambient ionization mass spectrometry imaging for disease diagnosis: Excitements and challenges.J Biosci. 2018 Sep;43(4):731-738. J Biosci. 2018. PMID: 30207318 Review.
Cited by
-
Prognostic value of serum CEA and CA19-9 levels in pancreatic ductal adenocarcinoma.Mol Clin Oncol. 2022 Jun 16;17(2):126. doi: 10.3892/mco.2022.2559. eCollection 2022 Aug. Mol Clin Oncol. 2022. PMID: 35832472 Free PMC article.
-
Mass spectrometry-based intraoperative tumor diagnostics.Future Sci OA. 2019 Mar 7;5(3):FSO373. doi: 10.4155/fsoa-2018-0087. eCollection 2019 Mar. Future Sci OA. 2019. PMID: 30906569 Free PMC article. Review.
-
Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry.Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):6700-6705. doi: 10.1073/pnas.1706459114. Epub 2017 Jun 12. Proc Natl Acad Sci U S A. 2017. PMID: 28607048 Free PMC article. Clinical Trial.
-
Validation of full-field optical coherence tomography in distinguishing malignant and benign tissue in resected pancreatic cancer specimens.PLoS One. 2017 Apr 17;12(4):e0175862. doi: 10.1371/journal.pone.0175862. eCollection 2017. PLoS One. 2017. PMID: 28414765 Free PMC article.
-
Analysis of human gliomas by swab touch spray-mass spectrometry: applications to intraoperative assessment of surgical margins and presence of oncometabolites.Analyst. 2017 Oct 23;142(21):4058-4066. doi: 10.1039/c7an01334e. Analyst. 2017. PMID: 28984323 Free PMC article.
References
-
- Shoup M, Bouvet M, Farnell M. Discussion. J Am Coll Surg. 2012;215:135–136. 10.1016/j.jamcollsurg.2012.04.011 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

