Standardization and quality control for high-dimensional mass cytometry studies of human samples
- PMID: 27575385
- PMCID: PMC5495108
- DOI: 10.1002/cyto.a.22935
Standardization and quality control for high-dimensional mass cytometry studies of human samples
Abstract
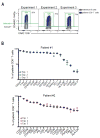
Mass cytometry (CyTOF), a mass spectrometry-based single cell phenotyping technology, allows utilization of over 35 antibodies in a single sample and is a promising tool for translational human immunology studies. Although several analysis tools are available to interpret the complex data sets generated, a robust method for standardization and quality control within and across studies is needed. Here we report an efficient and easily adaptable method to monitor quality of individual samples in human immunology studies and to facilitate reproducible data analysis. Samples to be assessed are spiked with a defined amount of reference peripheral blood mononuclear cells from a healthy donor, derived from a single large blood draw. The presence of known standardized numbers and phenotypic profiles of these reference cells greatly facilitates sample analysis by allowing for: 1) quality control for consistent staining of each antibody in the panel, 2) identification of potential batch effects, and 3) implementation of a robust gating strategy. We demonstrate the utility of this method using peripheral blood and bronchoalveolar lavage samples from HIV+ patients by characterizing their CD8+ T-cell phenotypes and cytokine expression, respectively. Our results indicate that this method allows quality control of experimental conditions and results in highly reproducible population frequencies through a robust gating strategy. © 2016 International Society for Advancement of Cytometry.
Keywords: CyTOF; HIV; Mass cytometry; clinical studies; human immunology.
© 2016 International Society for Advancement of Cytometry.
Figures


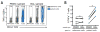
Comment in
-
High standards for high dimensional investigations.Cytometry A. 2016 Oct;89(10):886-888. doi: 10.1002/cyto.a.22992. Epub 2016 Sep 23. Cytometry A. 2016. PMID: 27662608 Free PMC article. No abstract available.
References
-
- Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, Pavlov S, Vorobiev S, Dick JE, Tanner SD. Mass cytometry: Technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2009;81:6813–6822. - PubMed
-
- Ornatsky O, Baranov VI, Bandura DR, Tanner SD, Dick J. Multiple cellular antigen detection by ICP-MS. J Immunol Methods. 2006;308:68–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

