Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma
- PMID: 27575820
- PMCID: PMC5124387
- DOI: 10.1053/j.gastro.2016.08.029
Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma
Abstract
Background & aims: Conventional transarterial chemoembolization (cTACE) is used to treat patients with hepatocellular carcinoma (HCC). Radioembolization is a minimally invasive procedure that involves implantation of radioactive micron-sized particles loaded with yttrium-90 (Y90) inside the blood vessels that supply a tumor. We performed a randomized, phase 2 study to compare the effects of cTACE and Y90 radioembolization in patients with HCC.
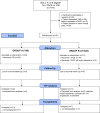
Methods: From October 2009 through October 2015, we reviewed patients with HCC of all Barcelona Clinic Liver Cancer (BCLC) stages for eligibility. Of these, 179 patients with BCLC stages A or B met our enrollment criteria and were candidates for cTACE or Y90 therapy. Patients were assigned randomly to groups that received Y90 therapy (n = 24; 50% Child-Pugh A) or cTACE (n = 21; 71% Child-Pugh A). The primary outcome was time to progression (TTP), evaluated by intention-to-treat analysis. Secondary outcomes included safety, rate of response (based on tumor size and necrosis criteria), and Kaplan-Meier survival time. We performed inverse probability of censoring weighting and competing risk analyses.
Results: Patients in the Y90 radioembolization group had significant longer median TTP (>26 mo) than patients in the cTACE group (6.8 mo; P = .0012) (hazard ratio, 0.122; 95% confidence interval [CI], 0.027-0.557; P = .007). This was confirmed by competing risk and inverse probability of censoring weighting analyses accounting for transplantation or death. A significantly greater proportion of patients in the cTACE group developed diarrhea (21%) than in the Y90 group (0%; P = .031) or hypoalbuminemia (58% in the cTACE group vs 4% in the Y90 group; P < .001). Similar proportions of patients in each group had a response to therapy, marked by necrosis (74% in the cTACE group vs 87% in the Y90 group) (P = .433). The median survival time, censored to liver transplantation, was 17.7 months for the cTACE group (95% CI, 8.3-not calculable) vs 18.6 months for the Y90 group (95% CI, 7.4-32.5) (P = .99).
Conclusions: In a randomized phase 2 study of patients with HCC of BCLC stages A or B, we found Y90 radioembolization to provide significantly longer TTP than cTACE. Y90 radioembolization provides better tumor control and could reduce drop-out from transplant waitlists. ClinicalTrials.gov no. NCT00956930.
Keywords: Chemoembolization; Liver Cancer; Radioembolization; Randomized Trial.
Copyright © 2016 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interest: RJL, LK, RS serve as advisors to BTG. None of the other authors report a conflict of interest.
Figures



Comment in
-
Transarterial Radioembolization for Hepatocellular Carcinoma: Who, When… and Y(90)?Gastroenterology. 2016 Dec;151(6):1062-1065. doi: 10.1053/j.gastro.2016.10.031. Epub 2016 Oct 27. Gastroenterology. 2016. PMID: 27983951 No abstract available.
-
Does Y90 Radioembolization Prolong Overall Survival Compared With Chemoembolization in Patients With Hepatocellular Carcinoma?Gastroenterology. 2017 May;152(6):1624-1625. doi: 10.1053/j.gastro.2017.01.061. Epub 2017 Mar 31. Gastroenterology. 2017. PMID: 28371622 No abstract available.
-
Regarding: Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma.Gastroenterology. 2017 May;152(6):1626-1627. doi: 10.1053/j.gastro.2016.09.070. Epub 2017 Mar 31. Gastroenterology. 2017. PMID: 28371623 No abstract available.
-
Reply.Gastroenterology. 2017 May;152(6):1628-1629. doi: 10.1053/j.gastro.2017.03.044. Epub 2017 Apr 1. Gastroenterology. 2017. PMID: 28376318 No abstract available.
-
RE: Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma.Gastroenterology. 2017 May;152(6):1625-1626. doi: 10.1053/j.gastro.2016.09.069. Epub 2017 Apr 1. Gastroenterology. 2017. PMID: 28376319 No abstract available.
-
Is Y90 Radioembolization Superior or Comparable to Transarterial Chemoembolization for Treating Hepatocellular Carcinoma?Gastroenterology. 2017 May;152(6):1627-1628. doi: 10.1053/j.gastro.2016.10.048. Epub 2017 Apr 4. Gastroenterology. 2017. PMID: 28384449 No abstract available.
-
Transarterial Chemoembolization vs Yttrium-90 Transarterial Radioembolization.Gastroenterology. 2017 May;152(6):1623-1624. doi: 10.1053/j.gastro.2017.01.062. Epub 2017 Apr 3. Gastroenterology. 2017. PMID: 28384457 No abstract available.
References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Hepatobiliary Cancers. 2016
-
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. - PubMed
-
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

