Comparing desferrioxamine and light fractionation enhancement of ALA-PpIX photodynamic therapy in skin cancer
- PMID: 27575852
- PMCID: PMC5046214
- DOI: 10.1038/bjc.2016.267
Comparing desferrioxamine and light fractionation enhancement of ALA-PpIX photodynamic therapy in skin cancer
Abstract
Background: Aminolevulinic acid (ALA)-based photodynamic therapy (PDT) provides selective uptake and conversion of ALA into protoporphyrin IX (PpIX) in actinic keratosis and squamous cell carcinoma, yet large response variations in effect are common between individuals. The aim of this study was to compare pre-treatment strategies that increase the therapeutic effect, including fractionated light delivery during PDT (fPDT) and use of iron chelator desferrioxamine (DFO), separately and combined.
Methods: Optical measurements of fluorescence were used to quantify PpIX produced, and the total amount of PpIX photobleached as an implicit measure of the photodynamic dose. In addition, measurements of white light reflectance were used to quantify changes in vascular physiology throughout the PDT treatment.
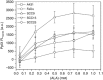
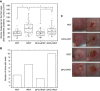
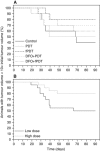
Results: fPDT produced both a replenishment of PpIX and vascular re-oxygenation during a 2 h dark interval between the first and second PDT light fractions. The absolute photodynamic dose was increased 57% by fPDT, DFO and their combination, as compared with PDT group (from 0.7 to 1.1). Despite that light fractionation increased oedema and scab formation during the week after treatment, no significant difference in long-term survival has been observed between treatment groups. However, outcomes stratified on the basis of measured photodynamic dose showed a significant difference in long-term survival.
Conclusions: The assessment of implicit photodynamic dose was a more significant predictor of efficacy for ALA-PDT skin cancer treatments than prescription of an enhanced treatment strategy, likely because of high individual variation in response between subjects.
Figures

Similar articles
-
The effect of an iron chelating agent on protoporphyrin IX levels and phototoxicity in topical 5-aminolaevulinic acid photodynamic therapy.Br J Dermatol. 2003 Jul;149(1):124-30. doi: 10.1046/j.1365-2133.2003.05351.x. Br J Dermatol. 2003. PMID: 12890205 Clinical Trial.
-
Desferrioxamine shows different potentials for enhancing 5-aminolaevulinic acid-based photodynamic therapy in several cutaneous cell lines.Lasers Med Sci. 2010 Mar;25(2):251-7. doi: 10.1007/s10103-009-0721-0. Epub 2009 Aug 25. Lasers Med Sci. 2010. PMID: 19705180
-
The effect of light fractionation with a 2-h dark interval on the efficacy of topical hexyl-aminolevulinate photodynamic therapy in normal mouse skin.Photodiagnosis Photodyn Ther. 2013 Dec;10(4):703-9. doi: 10.1016/j.pdpdt.2013.09.002. Epub 2013 Oct 15. Photodiagnosis Photodyn Ther. 2013. PMID: 24284130
-
Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement.Int J Mol Sci. 2015 Oct 28;16(10):25865-80. doi: 10.3390/ijms161025865. Int J Mol Sci. 2015. PMID: 26516850 Free PMC article. Review.
-
5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges.Cancer. 1997 Jun 15;79(12):2282-308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. Cancer. 1997. PMID: 9191516 Review.
Cited by
-
Exploiting a New Approach to Destroy the Barrier of Tumor Microenvironment: Nano-Architecture Delivery Systems.Molecules. 2021 May 5;26(9):2703. doi: 10.3390/molecules26092703. Molecules. 2021. PMID: 34062992 Free PMC article. Review.
-
Phototherapy in cancer treatment: strategies and challenges.Signal Transduct Target Ther. 2025 Apr 2;10(1):115. doi: 10.1038/s41392-025-02140-y. Signal Transduct Target Ther. 2025. PMID: 40169560 Free PMC article. Review.
-
Photosensitized Oxidative Damage from a New Perspective: The Influence of Before-Light and After-Light Reaction Conditions.J Org Chem. 2024 Sep 20;89(18):12873-12885. doi: 10.1021/acs.joc.4c01305. Epub 2024 Sep 4. J Org Chem. 2024. PMID: 39231123 Free PMC article. Review.
-
Effect of intermittency factor on singlet oxygen and PGE2 formation in azulene-mediated photodynamic therapy: A preliminary study.Biochem Biophys Rep. 2022 Jun 4;31:101290. doi: 10.1016/j.bbrep.2022.101290. eCollection 2022 Sep. Biochem Biophys Rep. 2022. PMID: 35677631 Free PMC article.
-
Probing Hexaminolevulinate Mediated PpIX Fluorescence in Cancer Cell Suspensions in the Presence of Chemical Adjuvants.Int J Mol Sci. 2020 Apr 22;21(8):2963. doi: 10.3390/ijms21082963. Int J Mol Sci. 2020. PMID: 32331454 Free PMC article.
References
-
- Allison RR, Sibata CH, Downie GH, Cuenca RE (2006) A clinical review of PDT for cutaneous malignancies. Photodiagnosis Photodyn Ther 3: 214–226. - PubMed
-
- Araújo LMPC, Thomazine JA, Lopez RFV (2010) Development of microemulsions to topically deliver 5-aminolevulinic acid in photodynamic therapy. Eur J Pharm Biopharm 75: 48–55. - PubMed
-
- Blake E, Curnow A (2010) The hydroxypyridinone iron chelator CP94 can enhance PpIX-induced PDT of cultured human glioma cells. Photochem Photobiol 86: 1154–1160. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

