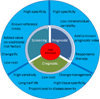
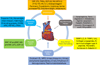
Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers
- PMID: 27576060
- PMCID: PMC5253084
- DOI: 10.1016/j.tcm.2016.07.005
Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers
Abstract
Cardiovascular disease (CVD) is a leading cause of death worldwide and continues to increase in prevalence compared to previous decades, in part because of the aging of the world population. Atherosclerotic CVD starts at a very young age and progresses over time allowing sufficient time for screening and early detection of the condition. Advances in biomarker research and developments related to CVD over the past 30 years have led to more sensitive screening methods, a greater emphasis on its early detection and diagnosis, and improved treatments resulting in more favorable clinical outcomes in the community. However, the use of biomarkers for different purposes in CVD remains an important area of research that has been explored by scientists over the years and many new developments are still underway. Therefore, a detailed description of all CVD biomarkers that are currently been used or investigated for future use in the field of cardiovascular medicine is out of scope for any review article. In the present review, we do not intend to replicate the information from previous exhaustive review on biomarkers, but highlight key statistical and clinical issues with an emphasis on methods to evaluate the incremental yield of biomarkers, including their clinical utility, a prerequisite before any putative novel biomarker is utilized in clinical practice. In addition, we will summarize information regarding recent novel heart failure biomarkers in current practice, which are undergoing scrutiny before they can be available for clinical use, and their impact on clinical outcomes.
Keywords: Biomarkers; Cardiovascular disease; Heart failure.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Editorial commentary: Biomarkers in heart failure, between scientific scrutiny and exploration.Trends Cardiovasc Med. 2017 Feb;27(2):134-135. doi: 10.1016/j.tcm.2016.08.004. Epub 2016 Aug 11. Trends Cardiovasc Med. 2017. PMID: 27666742 No abstract available.
References
-
- [accessed on July 23, 2016]; http://www.who.int/mediacentre/factsheets/fs310/en/
-
- Balagopal PB, de Ferranti SD, Cook S, Daniels SR, Gidding SS, Hayman LL, et al. Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation. 2011;123:2749–2769. - PubMed
-
- Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. - PubMed
-
- Thygesen K, Alpert JS, White HD, et al. on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction, TASK FORCE MEMBERS: Chairpersons: Kristian Thygesen (Denmark), Biomarker Group: Allan S. Jaffe CU. Universal Definition of Myocardial Infarction. Circulation. 2007;116:2634–2653. - PubMed
-
- Vasan RS. Biomarkers of Cardiovascular Disease: Molecular Basis and Practical Considerations. Circulation. 2006;113:2335–2362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous