Activation of Dioxygen by Iron and Manganese Complexes: A Heme and Nonheme Perspective
- PMID: 27576170
- PMCID: PMC5228556
- DOI: 10.1021/jacs.6b05251
Activation of Dioxygen by Iron and Manganese Complexes: A Heme and Nonheme Perspective
Abstract
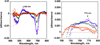
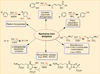
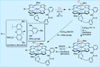
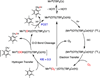
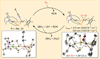
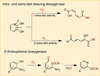
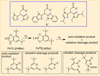
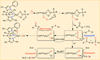
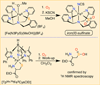
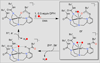
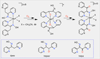
The rational design of well-defined, first-row transition metal complexes that can activate dioxygen has been a challenging goal for the synthetic inorganic chemist. The activation of O2 is important in part because of its central role in the functioning of metalloenzymes, which utilize O2 to perform a number of challenging reactions including the highly selective oxidation of various substrates. There is also great interest in utilizing O2, an abundant and environmentally benign oxidant, in synthetic catalytic oxidation systems. This Perspective brings together recent examples of biomimetic Fe and Mn complexes that can activate O2 in heme or nonheme-type ligand environments. The use of oxidants such as hypervalent iodine (e.g., ArIO), peracids (e.g., m-CPBA), peroxides (e.g., H2O2) or even superoxide is a popular choice for accessing well-characterized metal-superoxo, metal-peroxo, or metal-oxo species, but the instances of biomimetic Fe/Mn complexes that react with dioxygen to yield such observable metal-oxygen species are surprisingly few. This Perspective focuses on mononuclear Fe and Mn complexes that exhibit reactivity with O2 and lead to spectroscopically observable metal-oxygen species, and/or oxidize biologically relevant substrates. Analysis of these examples reveals that solvent, spin state, redox potential, external co-reductants, and ligand architecture can all play important roles in the O2 activation process.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

