Bioavailability, Efficacy and Safety of Generic Immunosuppressive Drugs for Kidney Transplantation: A Systematic Review and Meta-Analysis
- PMID: 27576318
- PMCID: PMC6584577
- DOI: 10.1159/000449020
Bioavailability, Efficacy and Safety of Generic Immunosuppressive Drugs for Kidney Transplantation: A Systematic Review and Meta-Analysis
Abstract
Background: Concerns exist over the extrapolation of bioavailability studies of generic immunosuppressive drugs in healthy volunteers, regarding their efficacy and safety in kidney transplant recipients. We conducted a meta-analysis of trials examining the bioavailability of generic (test) immunosuppressive drugs relative to their brand (reference) counterparts in healthy volunteers, based on the US Food and Drug Administration requirements for approval of generics, and their efficacy and safety in kidney transplant recipients.
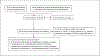
Methods: Eligible studies were identified in PubMed, Cochrane Central Register of Controlled Trials, Scopus, ClinicalTrials.gov, and conference abstracts.
Results: Twenty crossover trials of healthy volunteers (n = 641) and 6 parallel-arm randomized controlled trials of kidney transplant recipients (n = 594) were identified. The 90% CI of the pooled test-to-reference drug ratio for maximum or peak plasma concentration (Cmax) and area under the plasma concentration time-curve from time 0 to time of last determinable concentration (AUC(0-t)) fell within the required range (0.80-1.25) for cyclosporine (Cmax 0.91; 90% CI 0.86-0.95; and AUC(0-t) 0.97; 90% CI 0.94-1.00), tacrolimus (Cmax 1.17; 90% CI 1.09-1.24; and AUC(0-t) 1.00; 90% CI 0.97-1.03) and mycophenolate mofetil (Cmax 0.98; 90% CI 0.96-1.01; and AUC(0-t) 1.00; 90% CI 0.99-1.01). In subgroup analyses, some generic cyclosporine formulations did not meet criteria for bioequivalence. No significant differences were observed in the time to maximum plasma concentration and terminal plasma half-life between generic and brand drugs. In parallel-arm trials, generic cyclosporine was non-inferior to brand counterpart in terms of acute allograft rejection, infections, and death.
Conclusions: Not all generic immunosuppressive drugs have similar relative bioavailability to their brand name counterparts. Evidence on their efficacy and safety is inconclusive. Tighter regulatory requirement for approval of generic drugs with narrow therapeutic index is needed.
© 2016 S. Karger AG, Basel.
Conflict of interest statement
Disclosure Statement
The authors declare that they have no other relevant financial interests.
Figures




Similar articles
-
Non-biologic, steroid-sparing therapies for non-infectious intermediate, posterior, and panuveitis in adults.Cochrane Database Syst Rev. 2022 Oct 31;10(10):CD014831. doi: 10.1002/14651858.CD014831.pub2. Cochrane Database Syst Rev. 2022. PMID: 36315029 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Generic immunosuppression in solid organ transplantation: systematic review and meta-analysis.BMJ. 2015 Jun 22;350:h3163. doi: 10.1136/bmj.h3163. BMJ. 2015. PMID: 26101226 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.Health Technol Assess. 2005 May;9(21):1-179, iii-iv. doi: 10.3310/hta9210. Health Technol Assess. 2005. PMID: 15899149
Cited by
-
Bioequivalence trials for the approval of generic drugs in Saudi Arabia: a descriptive analysis of design aspects.BMC Med Res Methodol. 2024 Apr 5;24(1):82. doi: 10.1186/s12874-024-02207-4. BMC Med Res Methodol. 2024. PMID: 38580928 Free PMC article.
-
The Role of Regulatory Myeloid Cell Therapy in Renal Allograft Rejection.Front Immunol. 2021 Feb 24;12:625998. doi: 10.3389/fimmu.2021.625998. eCollection 2021. Front Immunol. 2021. PMID: 33717141 Free PMC article. Review.
-
Antitumor pharmacotherapy of colorectal cancer in kidney transplant recipients.Ther Adv Med Oncol. 2019 Sep 23;11:1758835919876196. doi: 10.1177/1758835919876196. eCollection 2019. Ther Adv Med Oncol. 2019. PMID: 31579127 Free PMC article. Review.
-
Meeting Regulatory Requirements for Drugs with a Narrow Therapeutic Index: Bioequivalence Studies of Generic Once-Daily Tacrolimus.Drug Healthc Patient Saf. 2020 Sep 8;12:151-160. doi: 10.2147/DHPS.S256455. eCollection 2020. Drug Healthc Patient Saf. 2020. PMID: 32982466 Free PMC article.
-
Original and generic preservation solutions in organ transplantation. A new paradigm?Acta Cir Bras. 2020 Mar 9;35(1):e202000101. doi: 10.1590/s0102-865020200010000001. eCollection 2020. Acta Cir Bras. 2020. PMID: 32159587 Free PMC article.
References
-
- US Renal Data System: USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States: National Institutes of Health. Bethesda, National Institute of Diabetes and Digestive and Kidney Diseases, 2013.
-
- Gill JS, Tonelli M: Penny wise, pound foolish? Coverage limits on immunosuppression after kidney transplantation. N Engl J Med 2012; 366:586–589. - PubMed
-
- Denhaerynck K, Dobbels F, Cleemput I, et al.: Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int 2005; 18: 1121–1133. - PubMed
-
- Nevins TE, Kruse L, Skeans MA, Thomas W: The natural history of azathioprine compliance after renal transplantation. Kidney Int 2001;60:1565–1570. - PubMed
-
- Generic Pharmaceutical Association: Annual Report. 2014. http://www.gphaonline.org/ (accessed March 25, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

