Combination Therapy of Etanercept and Fumarates versus Etanercept Monotherapy in Psoriasis: A Randomized Exploratory Study
- PMID: 27576483
- PMCID: PMC5296929
- DOI: 10.1159/000448135
Combination Therapy of Etanercept and Fumarates versus Etanercept Monotherapy in Psoriasis: A Randomized Exploratory Study
Abstract
Background: Biologics are a safe and efficacious therapy for psoriasis. The drug survival of biologics may be disappointing, primarily due to loss of efficacy. Therefore, safe combination treatments are sought to improve their clinical response.
Objective: To assess the efficacy, safety and tolerability of the combination therapy of etanercept with fumarates versus etanercept monotherapy.
Methods: Thirty-three patients with psoriasis were randomized 1:1 to receive etanercept combined with fumarates or etanercept monotherapy. The primary outcome measure was the difference in PASI-75 response after 24 weeks; additionally, a longitudinal analysis was performed. An important secondary outcome measure was the proportion of patients with a Physician Global Assessment (PGA) of clear or almost clear. Adverse events were collected throughout the study.
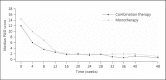
Results: In the combination therapy group, 78% (14 out of 18 patients) reached PASI-75 at week 24 versus 57% (8 out of 14 patients) in the monotherapy group (p = 0.27). The longitudinal analysis showed a PASI reduction of 5.97% per week for the combination therapy group and of 4.76% for the monotherapy group (p = 0.11). In the combination therapy group, 94% (17 out of 18 patients) of patients had a PGA of clear/almost clear versus 64% (9 out of 14 patients) in the monotherapy group (p = 0.064). The incidence of mild gastrointestinal complaints was higher in the combination group than in the monotherapy group.
Conclusion: Using the PGA, combination therapy showed a trend towards faster improvement in the first 24 weeks. The difference in the PASI score between the two groups was not statistically significant. Addition of fumarates to etanercept for 48 weeks appeared safe with an acceptable tolerability.
© 2016 S. Karger AG, Basel.
Figures
References
-
- Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol. 2001;15:16–17. - PubMed
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–407. - PubMed
-
- Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–139. - PubMed
-
- Mrowietz U, de Jong EM, Kragballe K, Langley R, Nast A, Puig L, Reich K, Schmitt J, Warren RB. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28:438–453. - PubMed
-
- Zandvliet ML, van Bezooijen JS, Bos MA, Prens EP, van Doorn M, Bijen I, Schreurs MW, van der Velden VH, Koch BC, van Gelder T. Monitoring antigen-specific biologics: current knowledge and future prospects. Ther Drug Monit. 2013;35:588–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

