The pesticidal Cry6Aa toxin from Bacillus thuringiensis is structurally similar to HlyE-family alpha pore-forming toxins
- PMID: 27576487
- PMCID: PMC5004264
- DOI: 10.1186/s12915-016-0295-9
The pesticidal Cry6Aa toxin from Bacillus thuringiensis is structurally similar to HlyE-family alpha pore-forming toxins
Abstract
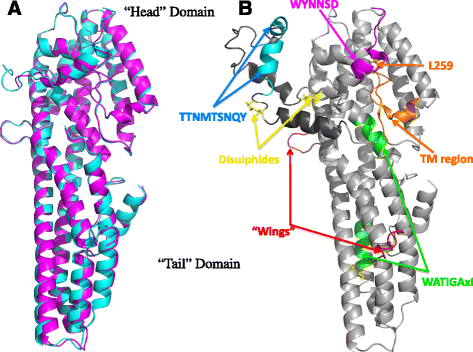
Background: The Cry6 family of proteins from Bacillus thuringiensis represents a group of powerful toxins with great potential for use in the control of coleopteran insects and of nematode parasites of importance to agriculture. These proteins are unrelated to other insecticidal toxins at the level of their primary sequences and the structure and function of these proteins has been poorly studied to date. This has inhibited our understanding of these toxins and their mode of action, along with our ability to manipulate the proteins to alter their activity to our advantage. To increase our understanding of their mode of action and to facilitate further development of these proteins we have determined the structure of Cry6Aa in protoxin and trypsin-activated forms and demonstrated a pore-forming mechanism of action.
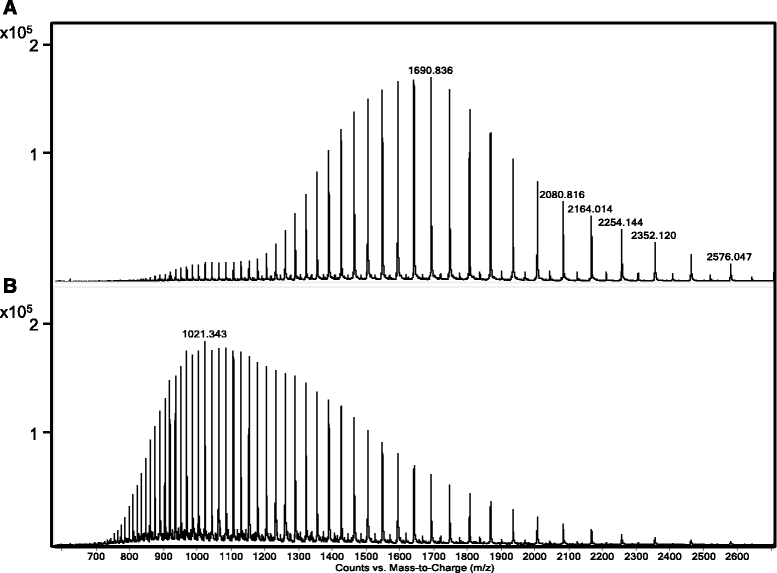
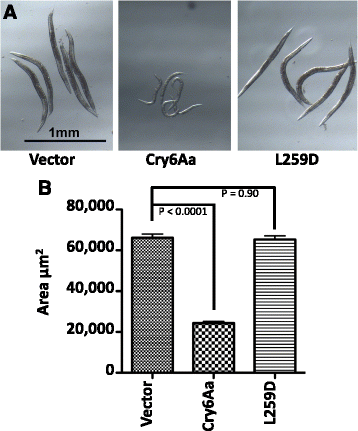
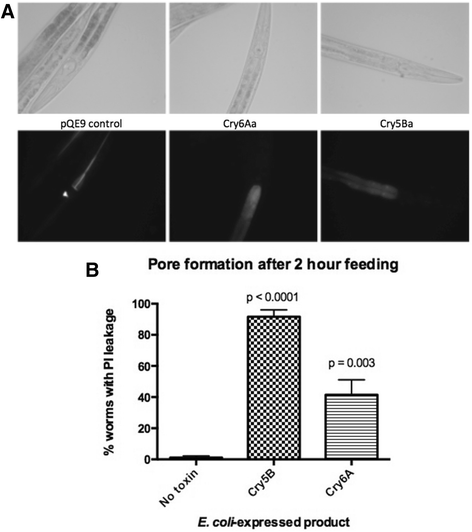
Results: The two forms of the toxin were resolved to 2.7 Å and 2.0 Å respectively and showed very similar structures. Cry6Aa shows structural homology to a known class of pore-forming toxins including hemolysin E from Escherichia coli and two Bacillus cereus proteins: the hemolytic toxin HblB and the NheA component of the non-hemolytic toxin (pfam05791). Cry6Aa also shows atypical features compared to other members of this family, including internal repeat sequences and small loop regions within major alpha helices. Trypsin processing was found to result in the loss of some internal sequences while the C-terminal region remains disulfide-linked to the main core of the toxin. Based on the structural similarity of Cry6Aa to other toxins, the mechanism of action of the toxin was probed and its ability to form pores in vivo in Caenorhabditis elegans was demonstrated. A non-toxic mutant was also produced, consistent with the proposed pore-forming mode of action.
Conclusions: Cry6 proteins are members of the alpha helical pore-forming toxins - a structural class not previously recognized among the Cry toxins of B. thuringiensis and representing a new paradigm for nematocidal and insecticidal proteins. Elucidation of both the structure and the pore-forming mechanism of action of Cry6Aa now opens the way to more detailed analysis of toxin specificity and the development of new toxin variants with novel activities.
Keywords: Bacillus thuringiensis; Cry6; Hemolysin; Insecticidal toxin.
Figures



Similar articles
-
Crystal structure of Cry6Aa: A novel nematicidal ClyA-type α-pore-forming toxin from Bacillus thuringiensis.Biochem Biophys Res Commun. 2016 Sep 9;478(1):307-313. doi: 10.1016/j.bbrc.2016.07.002. Epub 2016 Jul 2. Biochem Biophys Res Commun. 2016. PMID: 27381865
-
Crystal structure of Cry51Aa1: A potential novel insecticidal aerolysin-type β-pore-forming toxin from Bacillus thuringiensis.Biochem Biophys Res Commun. 2015 Jul 3;462(3):184-9. doi: 10.1016/j.bbrc.2015.04.068. Epub 2015 May 7. Biochem Biophys Res Commun. 2015. PMID: 25957471
-
Binding of Cyt1Aa and Cry11Aa toxins of Bacillus thuringiensis serovar israelensis to brush border membrane vesicles of Tipula paludosa (Diptera: Nematocera) and subsequent pore formation.Appl Environ Microbiol. 2007 Jun;73(11):3623-9. doi: 10.1128/AEM.01056-06. Epub 2007 Apr 6. Appl Environ Microbiol. 2007. PMID: 17416690 Free PMC article.
-
Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins.Toxins (Basel). 2014 Sep 16;6(9):2732-70. doi: 10.3390/toxins6092732. Toxins (Basel). 2014. PMID: 25229189 Free PMC article. Review.
-
Pore formation by Cry toxins.Adv Exp Med Biol. 2010;677:127-42. doi: 10.1007/978-1-4419-6327-7_11. Adv Exp Med Biol. 2010. PMID: 20687486 Review.
Cited by
-
Novel Nematode-Killing Protein-1 (Nkp-1) from a Marine Epiphytic Bacterium Pseudoalteromonas tunicata.Biomedicines. 2021 Oct 30;9(11):1586. doi: 10.3390/biomedicines9111586. Biomedicines. 2021. PMID: 34829814 Free PMC article.
-
Cry6Aa1, a Bacillus thuringiensis nematocidal and insecticidal toxin, forms pores in planar lipid bilayers at extremely low concentrations and without the need of proteolytic processing.J Biol Chem. 2017 Aug 11;292(32):13122-13132. doi: 10.1074/jbc.M116.765941. Epub 2017 Jun 16. J Biol Chem. 2017. PMID: 28623231 Free PMC article.
-
Resistance to Cry14A family Bacillus thuringiensis crystal proteins in Caenornabditis elegans operates via the nhr-31 transcription factor and vacuolar-type ATPase pathway.PLoS Pathog. 2024 Oct 18;20(10):e1012611. doi: 10.1371/journal.ppat.1012611. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39423230 Free PMC article.
-
In Silico Functional Annotation and Structural Characterization of Hypothetical Proteins in Bacillus paralicheniformis and Bacillus subtilis Isolated from Honey.ACS Omega. 2025 Feb 27;10(9):8993-9006. doi: 10.1021/acsomega.4c07105. eCollection 2025 Mar 11. ACS Omega. 2025. PMID: 40092810 Free PMC article.
-
Multiple Horizontal Transfers of Immune Genes Between Distantly Related Teleost Fishes.Mol Biol Evol. 2025 Apr 30;42(5):msaf107. doi: 10.1093/molbev/msaf107. Mol Biol Evol. 2025. PMID: 40378191 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

