Human Sex Determination at the Edge of Ambiguity: INHERITED XY SEX REVERSAL DUE TO ENHANCED UBIQUITINATION AND PROTEASOMAL DEGRADATION OF A MASTER TRANSCRIPTION FACTOR
- PMID: 27576690
- PMCID: PMC5063998
- DOI: 10.1074/jbc.M116.741959
Human Sex Determination at the Edge of Ambiguity: INHERITED XY SEX REVERSAL DUE TO ENHANCED UBIQUITINATION AND PROTEASOMAL DEGRADATION OF A MASTER TRANSCRIPTION FACTOR
Abstract
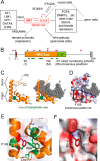
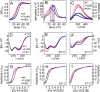
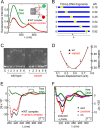
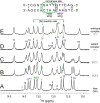
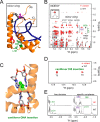
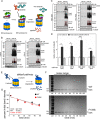
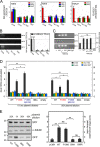
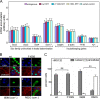
A general problem is posed by analysis of transcriptional thresholds governing cell fate decisions in metazoan development. A model is provided by testis determination in therian mammals. Its key step, Sertoli cell differentiation in the embryonic gonadal ridge, is initiated by SRY, a Y-encoded architectural transcription factor. Mutations in human SRY cause gonadal dysgenesis leading to XY female development (Swyer syndrome). Here, we have characterized an inherited mutation compatible with either male or female somatic phenotypes as observed in an XY father and XY daughter, respectively. The mutation (a crevice-forming substitution at a conserved back surface of the SRY high mobility group box) markedly destabilizes the domain but preserves specific DNA affinity and induced DNA bend angle. On transient transfection of diverse human and rodent cell lines, the variant SRY exhibited accelerated proteasomal degradation (relative to wild type) associated with increased ubiquitination; in vitro susceptibility to ubiquitin-independent ("default") cleavage by the 20S core proteasome was unchanged. The variant's gene regulatory activity (as assessed in a cellular model of the rat embryonic XY gonadal ridge) was reduced by 2-fold relative to wild-type SRY at similar levels of mRNA expression. Chemical proteasome inhibition restored native-like SRY expression and transcriptional activity in association with restored occupancy of a sex-specific enhancer element in principal downstream gene Sox9, demonstrating that the variant SRY exhibits essentially native activity on a per molecule basis. Our findings define a novel mechanism of impaired organogenesis, accelerated ubiquitin-directed proteasomal degradation of a master transcription factor leading to a developmental decision poised at the edge of ambiguity.
Keywords: DNA-binding domain; Sywer syndrome; developmental factor; disorders of sexual development; intersex; proteasome; protein degradation; reproduction; transcription.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Comment in
-
The Fate of Sex: SRY on the Ridge.Biol Reprod. 2016 Nov;95(5):106. doi: 10.1095/biolreprod.116.145342. Epub 2016 Sep 28. Biol Reprod. 2016. PMID: 27856452 No abstract available.
References
-
- Atkinson M. R., Savageau M. A., Myers J. T., and Ninfa A. J. (2003) Development of genetic circuitry exhibiting toggle switch or oscillatory behavior in Escherichia coli. Cell 113, 597–607 - PubMed
-
- Johnson A. D., Poteete A. R., Lauer G., Sauer R. T., Ackers G. K., and Ptashne M. (1981) λ repressor and cro—components of an efficient molecular switch. Nature 294, 217–223 - PubMed
-
- Ptashne M. (2004) A Genetic Switch: Phage λ Revisited, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
-
- Meyer B. J., Maurer R., and Ptashne M. (1980) Gene regulation at the right operator (O R) of bacteriophage λ: II. OR1, OR2, and OR3: Their roles in mediating the effects of repressor and cro. J. Mol. Biol. 139, 163–194 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

