Structural aspects of HDAC8 mechanism and dysfunction in Cornelia de Lange syndrome spectrum disorders
- PMID: 27576763
- PMCID: PMC5079251
- DOI: 10.1002/pro.3030
Structural aspects of HDAC8 mechanism and dysfunction in Cornelia de Lange syndrome spectrum disorders
Abstract
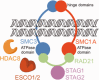
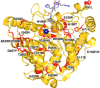
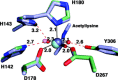
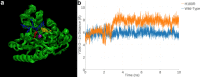
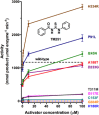
Cornelia de Lange Syndrome (CdLS) encompasses a broad spectrum of phenotypes characterized by distinctive craniofacial abnormalities, limb malformations, growth retardation, and intellectual disability. CdLS spectrum disorders are referred to as cohesinopathies, with ∼70% of patients having a mutation in a gene encoding a core cohesin protein (SMC1A, SMC3, or RAD21) or a cohesin regulatory protein (NIPBL or HDAC8). Notably, the regulatory function of HDAC8 in cohesin biology has only recently been discovered. This Zn2+ -dependent hydrolase catalyzes the deacetylation of SMC3, a necessary step for cohesin recycling during the cell cycle. To date, 23 different missense mutants in the gene encoding HDAC8 have been identified in children with developmental features that overlap those of CdLS. Enzymological, biophysical, and structural studies of CdLS HDAC8 protein mutants have yielded critical insight on compromised catalysis in vitro. Most CdLS HDAC8 mutations trigger structural changes that directly or indirectly impact substrate binding and catalysis. Additionally, several mutations significantly compromise protein thermostability. Intriguingly, catalytic activity in many HDAC8 mutants can be partially or fully restored by an N-acylthiourea activator, suggesting a plausible strategy for the chemical rescue of compromised HDAC8 catalysis in vivo.
Keywords: birth defect; human genetics; lysine deacetylase; protein crystallography; zinc enzyme.
© 2016 The Protein Society.
Figures

Similar articles
-
Biochemical and structural characterization of HDAC8 mutants associated with Cornelia de Lange syndrome spectrum disorders.Biochemistry. 2015 Oct 27;54(42):6501-13. doi: 10.1021/acs.biochem.5b00881. Epub 2015 Oct 14. Biochemistry. 2015. PMID: 26463496 Free PMC article.
-
Compromised structure and function of HDAC8 mutants identified in Cornelia de Lange Syndrome spectrum disorders.ACS Chem Biol. 2014 Sep 19;9(9):2157-64. doi: 10.1021/cb5003762. Epub 2014 Jul 30. ACS Chem Biol. 2014. PMID: 25075551 Free PMC article.
-
HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle.Nature. 2012 Sep 13;489(7415):313-7. doi: 10.1038/nature11316. Nature. 2012. PMID: 22885700 Free PMC article.
-
The expanding phenotypes of cohesinopathies: one ring to rule them all!Cell Cycle. 2019 Nov;18(21):2828-2848. doi: 10.1080/15384101.2019.1658476. Epub 2019 Sep 13. Cell Cycle. 2019. PMID: 31516082 Free PMC article. Review.
-
Mutation spectrum and genotype-phenotype correlation in Cornelia de Lange syndrome.Hum Mutat. 2013 Dec;34(12):1589-96. doi: 10.1002/humu.22430. Epub 2013 Sep 16. Hum Mutat. 2013. PMID: 24038889 Free PMC article. Review.
Cited by
-
The emerging role of chromatin remodelers in neurodevelopmental disorders: a developmental perspective.Cell Mol Life Sci. 2021 Mar;78(6):2517-2563. doi: 10.1007/s00018-020-03714-5. Epub 2020 Dec 2. Cell Mol Life Sci. 2021. PMID: 33263776 Free PMC article. Review.
-
Intrinsic structural dynamics dictate enzymatic activity and inhibition.Proc Natl Acad Sci U S A. 2023 Oct 10;120(41):e2310910120. doi: 10.1073/pnas.2310910120. Epub 2023 Oct 2. Proc Natl Acad Sci U S A. 2023. PMID: 37782780 Free PMC article.
-
Preparation of a new construct of human histone deacetylase 8 for the crystallization of enzyme-inhibitor complexes.Methods Enzymol. 2019;626:561-585. doi: 10.1016/bs.mie.2019.06.029. Epub 2019 Jul 18. Methods Enzymol. 2019. PMID: 31606091 Free PMC article.
-
X-Linked Sensorineural Hearing Loss: A Literature Review.Curr Genomics. 2018 Aug;19(5):327-338. doi: 10.2174/1389202919666171218163046. Curr Genomics. 2018. PMID: 30065609 Free PMC article. Review.
-
A Novel Intragenic Duplication in the HDAC8 Gene Underlying a Case of Cornelia de Lange Syndrome.Genes (Basel). 2022 Aug 8;13(8):1413. doi: 10.3390/genes13081413. Genes (Basel). 2022. PMID: 36011323 Free PMC article.
References
-
- Liu J, Baynam G (2010) Cornelia de Lange syndrome. Adv Exp Med Biol 685:111–123. - PubMed
-
- Boyle MI, Jespersgaard C, Brøndum‐Nielsen K, Bisgaard A‐M, Tümer Z (2015) Cornelia de Lange syndrome. Clin Genet 88:1–12. - PubMed
-
- Barisic I, Tokic V, Loane M, Bianchi F, Calzolari E, Garne E, Wellesley D, Dolk H, EUROCAT Working Group (2008) Descriptive epidemiology of Cornelia de Lange syndrome in Europe. Am J Med Genet a 146A:51–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

