Doxorubicin Conjugated to Immunomodulatory Anticancer Lactoferrin Displays Improved Cytotoxicity Overcoming Prostate Cancer Chemo resistance and Inhibits Tumour Development in TRAMP Mice
- PMID: 27576789
- PMCID: PMC5005995
- DOI: 10.1038/srep32062
Doxorubicin Conjugated to Immunomodulatory Anticancer Lactoferrin Displays Improved Cytotoxicity Overcoming Prostate Cancer Chemo resistance and Inhibits Tumour Development in TRAMP Mice
Erratum in
-
Author Correction: Doxorubicin Conjugated to Immunomodulatory Anticancer Lactoferrin Displays Improved Cytotoxicity Overcoming Prostate Cancer Chemo resistance and Inhibits Tumour Development in TRAMP Mice.Sci Rep. 2019 Apr 25;9(1):6529. doi: 10.1038/s41598-019-42459-5. Sci Rep. 2019. PMID: 31024014 Free PMC article.
Abstract
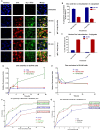
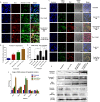
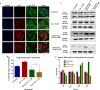
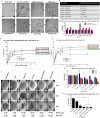
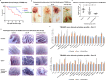
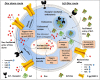
Advanced, metastatic, castration resistant and chemo-resistant prostate cancer has triggered change in the drug development landscape against prostate cancer. Bovine lactoferrin (bLf) is currently attracting attention in clinics for its anti-cancer properties and proven safety profile. bLf internalises into cancer cells via receptor mediated endocytosis, boosts immunity and complements chemotherapy. We employed bLf as an excellent functional carrier protein for delivering doxorubicin (Dox) into DU145 cells, CD44+/EpCAM+ double positive enriched DU145 3D prostaspheres and drug resistant ADR1000-DU145 cells, thus circumventing Dox efflux, to overcome chemo-resistance. Successful bLf-Dox conjugation with iron free or iron saturated bLf forms did not affect the integrity and functionality of bLf and Dox. bLf-Dox internalised into DU145 cells within 6 h, enhanced nuclear Dox retention up to 24 h, and proved significantly effective (p < 0.001) in reducing LC50 value of Dox from 5.3 μM to 1.3 μM (4 fold). Orally fed iron saturated bLf-Dox inhibited tumour development, prolonged survival, reduced Dox induced general toxicity, cardiotoxicity, neurotoxicity in TRAMP mice and upregulated serum levels of anti-cancer molecules TNF-α, IFN-γ, CCL4 and CCL17. The study identifies promising potential of a novel and safer bLf-Dox conjugate containing a conventional cytotoxic drug along with bLf protein to target drug resistance.
Figures

Similar articles
-
'Iron-saturated' lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy.Immunol Cell Biol. 2008 Mar-Apr;86(3):277-88. doi: 10.1038/sj.icb.7100163. Epub 2008 Feb 12. Immunol Cell Biol. 2008. PMID: 18268518
-
Oral administration of iron-saturated bovine lactoferrin-loaded ceramic nanocapsules for breast cancer therapy and influence on iron and calcium metabolism.Int J Nanomedicine. 2015 Jun 22;10:4081-98. doi: 10.2147/IJN.S75877. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26124661 Free PMC article.
-
LNA aptamer based multi-modal, Fe3O4-saturated lactoferrin (Fe3O4-bLf) nanocarriers for triple positive (EpCAM, CD133, CD44) colon tumor targeting and NIR, MRI and CT imaging.Biomaterials. 2015 Dec;71:84-99. doi: 10.1016/j.biomaterials.2015.07.055. Epub 2015 Aug 4. Biomaterials. 2015. PMID: 26318819
-
Clinical research review: usefulness of bovine lactoferrin in child health.Biometals. 2023 Jun;36(3):473-489. doi: 10.1007/s10534-022-00430-4. Epub 2022 Aug 9. Biometals. 2023. PMID: 35941293 Free PMC article. Review.
-
The Multifaceted Roles of Bovine Lactoferrin: Molecular Structure, Isolation Methods, Analytical Characteristics, and Biological Properties.J Agric Food Chem. 2023 Dec 27;71(51):20500-20531. doi: 10.1021/acs.jafc.3c06887. Epub 2023 Dec 13. J Agric Food Chem. 2023. PMID: 38091520 Free PMC article. Review.
Cited by
-
Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand.Biomaterials. 2020 Dec;263:120355. doi: 10.1016/j.biomaterials.2020.120355. Epub 2020 Sep 9. Biomaterials. 2020. PMID: 32932142 Free PMC article. Review.
-
Beyond Formulation: Contributions of Nanotechnology for Translation of Anticancer Natural Products into New Drugs.Pharmaceutics. 2022 Aug 17;14(8):1722. doi: 10.3390/pharmaceutics14081722. Pharmaceutics. 2022. PMID: 36015347 Free PMC article. Review.
-
The related miRNAs involved in doxorubicin resistance or sensitivity of various cancers: an update.Cancer Chemother Pharmacol. 2021 Nov;88(5):771-793. doi: 10.1007/s00280-021-04337-8. Epub 2021 Sep 12. Cancer Chemother Pharmacol. 2021. PMID: 34510251 Review.
-
Augmenting apoptosis-mediated anticancer activity of lactoperoxidase and lactoferrin by nanocombination with copper and iron hybrid nanometals.Sci Rep. 2022 Aug 1;12(1):13153. doi: 10.1038/s41598-022-17357-y. Sci Rep. 2022. PMID: 35915221 Free PMC article.
-
Efficiency of novel nanocombinations of bovine milk proteins (lactoperoxidase and lactoferrin) for combating different human cancer cell lines.Sci Rep. 2017 Dec 1;7(1):16769. doi: 10.1038/s41598-017-16962-6. Sci Rep. 2017. PMID: 29196676 Free PMC article.
References
-
- Yagoda A. & Petrylak D. Cytotoxic chemotherapy for advanced hormone‐resistant prostate cancer. Cancer 71, 1098–1109 (1993). - PubMed
-
- Tewey K., Rowe T., Yang L., Halligan B. & Liu L. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 226, 466–468 (1984). - PubMed
-
- Torti F. M. et al.. Weekly doxorubicin in endocrine-refractory carcinoma of the prostate. Journal of Clinical Oncology 1, 477–482 (1983). - PubMed
-
- Sella A. et al.. Phase II study of ketoconazole combined with weekly doxorubicin in patients with androgen-independent prostate cancer. Journal of Clinical Oncology 12, 683–688 (1994). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

