Mapping autoantigen epitopes: molecular insights into autoantibody-associated disorders of the nervous system
- PMID: 27577085
- PMCID: PMC5006540
- DOI: 10.1186/s12974-016-0678-4
Mapping autoantigen epitopes: molecular insights into autoantibody-associated disorders of the nervous system
Abstract
Background: Our knowledge of autoantibody-associated diseases of the central (CNS) and peripheral (PNS) nervous systems has expanded greatly over the recent years. A number of extracellular and intracellular autoantigens have been identified, and there is no doubt that this field will continue to expand as more autoantigens are discovered as a result of improved clinical awareness and methodological practice. In recent years, interest has shifted to uncover the target epitopes of these autoantibodies.
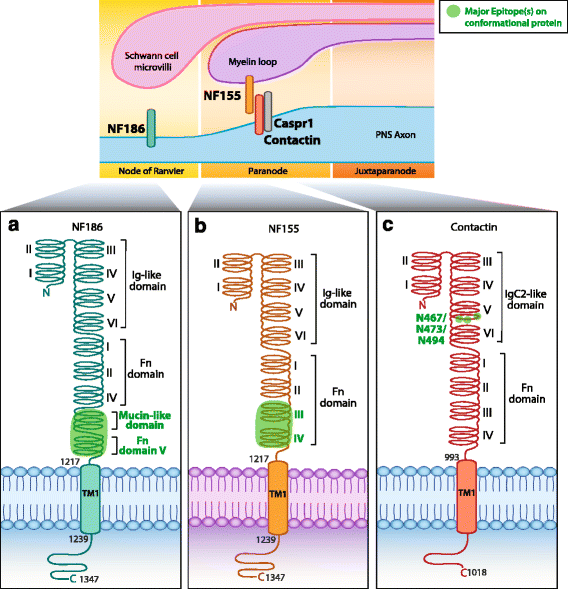
Main body: The purpose of this review is to discuss the mapping of the epitope targets of autoantibodies in CNS and PNS antibody-mediated disorders, such as N-methyl-D-aspartate receptor (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), leucine-rich glioma-inactivated protein 1 (Lgi1), contactin-associated protein-like 2 (Caspr2), myelin oligodendrocyte glycoprotein (MOG), aquaporin-4 (AQP4), 65 kDa glutamic acid decarboxylase (GAD65), acetylcholine receptor (AChR), muscle-specific kinase (MuSK), voltage-gated calcium channel (VGCC), neurofascin (NF), and contactin. We also address the methods used to analyze these epitopes, the relevance of their determination, and how this knowledge can inform studies on autoantibody pathogenicity. Furthermore, we discuss triggers of autoimmunity, such as molecular mimicry, ectopic antigen expression, epitope spreading, and potential mechanisms for the rising number of double autoantibody-positive patients.
Conclusions: Molecular insights into specificity and role of autoantibodies will likely improve diagnosis and treatment of CNS and PNS neuroimmune diseases.
Keywords: Autoantibody; CNS disorders; Epitope mapping; Epitope spreading; PNS disorders.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

