Endo-lysosomal TRP mucolipin-1 channels trigger global ER Ca2+ release and Ca2+ influx
- PMID: 27577094
- PMCID: PMC5087663
- DOI: 10.1242/jcs.190322
Endo-lysosomal TRP mucolipin-1 channels trigger global ER Ca2+ release and Ca2+ influx
Abstract
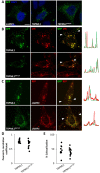
Transient receptor potential (TRP) mucolipins (TRPMLs), encoded by the MCOLN genes, are patho-physiologically relevant endo-lysosomal ion channels crucial for membrane trafficking. Several lines of evidence suggest that TRPMLs mediate localised Ca2+ release but their role in Ca2+ signalling is not clear. Here, we show that activation of endogenous and recombinant TRPMLs with synthetic agonists evoked global Ca2+ signals in human cells. These signals were blocked by a dominant-negative TRPML1 construct and a TRPML antagonist. We further show that, despite a predominant lysosomal localisation, TRPML1 supports both Ca2+ release and Ca2+ entry. Ca2+ release required lysosomal and ER Ca2+ stores suggesting that TRPMLs, like other endo-lysosomal Ca2+ channels, are capable of 'chatter' with ER Ca2+ channels. Our data identify new modalities for TRPML1 action.
Keywords: Ca2+; Endoplasmic reticulum; Lysosomes; TRP channels.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

