Phase 1 randomized controlled trial to evaluate the safety and immunogenicity of recombinant Pichia pastoris-expressed Plasmodium falciparum apical membrane antigen 1 (PfAMA1-FVO [25-545]) in healthy Malian adults in Bandiagara
- PMID: 27577237
- PMCID: PMC5006270
- DOI: 10.1186/s12936-016-1466-4
Phase 1 randomized controlled trial to evaluate the safety and immunogenicity of recombinant Pichia pastoris-expressed Plasmodium falciparum apical membrane antigen 1 (PfAMA1-FVO [25-545]) in healthy Malian adults in Bandiagara
Abstract
Background: The safety and immunogenicity of PfAMA1, adjuvanted with Alhydrogel(®) was assessed in malaria-experienced Malian adults. The malaria vaccine, PfAMA1-FVO [25-545] is a recombinant protein Pichia pastoris-expressed AMA-1 from Plasmodium falciparum FVO clone adsorbed to Alhydrogel(®), the control vaccine was tetanus toxoid produced from formaldehyde detoxified and purified tetanus toxin.
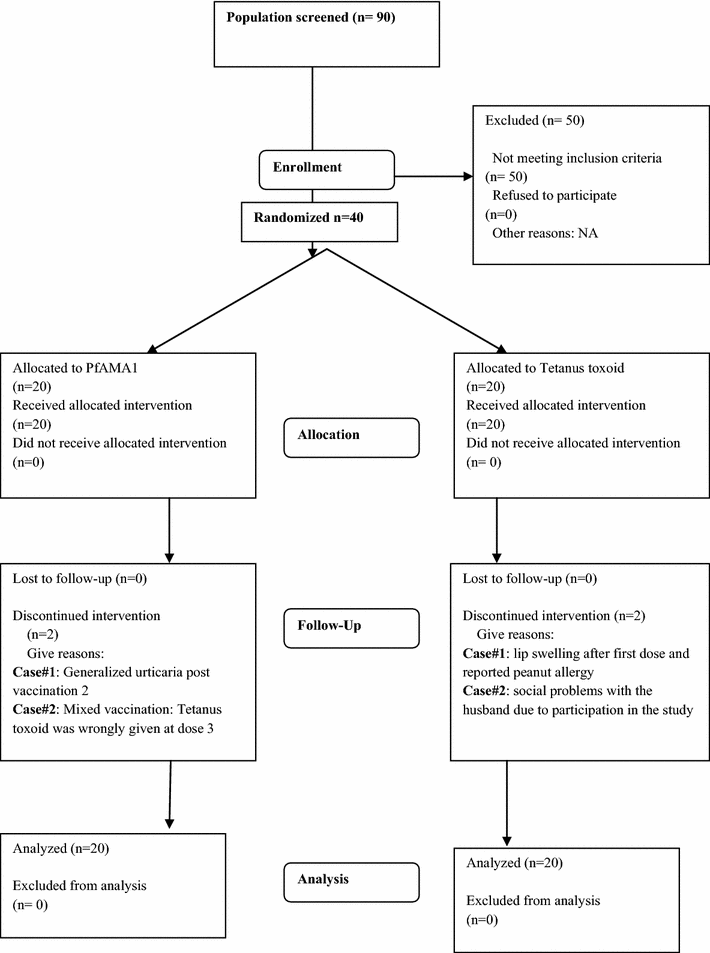
Methods: A double blind randomized controlled phase 1 study enrolled and followed 40 healthy adults aged 18-55 years in Bandiagara, Mali, West Africa, a rural setting with intense seasonal transmission of P. falciparum malaria. Volunteers were randomized to receive either 50 µg of malaria vaccine or the control vaccine. Three doses of vaccine were given on Days 0, 28 and 56, and participants were followed for 1 year. Solicited symptoms were assessed for seven days and unsolicited symptoms for 28 days after each vaccination. Serious adverse events were assessed throughout the study. The titres of anti-AMA-1 antibodies were measured by ELISA and P. falciparum growth inhibition assays were performed.
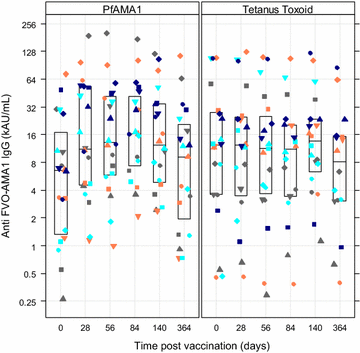
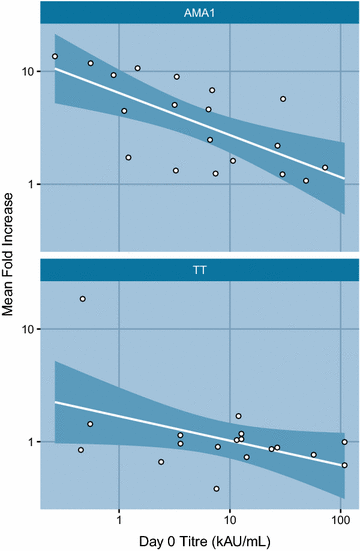
Results: Commonest local solicited adverse events were the injection site pain and swelling more frequent in the PfAMA1 group. No vaccine related serious adverse events were reported. A significant 3.5-fold increase of anti-AMA-1 IgG antibodies was observed in malaria vaccine recipients four weeks after the third immunization compared to the control group.
Conclusion: The PfAMA1 showed a good safety profile. Most adverse events reported were of mild to moderate intensity. In addition, the vaccine induced a significant though short-lived increase in the anti-AMA1 IgG titres. Registered on www.clinicaltrials.gov with the number NCT00431808.
Keywords: Blood-stage; Immunogenicity; Malaria; PfAMA1; Plasmodium falciparum antigen; Safety; Vaccine.
Figures
Similar articles
-
Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® in European and African adults: A phase 1a/1b, randomized, double-blind multi-centre trial.Vaccine. 2017 Oct 27;35(45):6218-6227. doi: 10.1016/j.vaccine.2017.09.027. Epub 2017 Sep 22. Vaccine. 2017. PMID: 28947345 Clinical Trial.
-
Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial.PLoS One. 2010 Feb 4;5(2):e9041. doi: 10.1371/journal.pone.0009041. PLoS One. 2010. PMID: 20140214 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial.PLoS One. 2008 Jan 23;3(1):e1465. doi: 10.1371/journal.pone.0001465. PLoS One. 2008. PMID: 18213374 Free PMC article. Clinical Trial.
-
Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum.Hum Vaccin. 2010 Jan;6(1):39-53. doi: 10.4161/hv.6.1.10712. Epub 2010 Jan 19. Hum Vaccin. 2010. PMID: 20061790 Review.
-
Pfs230: from malaria transmission-blocking vaccine candidate toward function.Parasite Immunol. 2003 Jul;25(7):351-9. doi: 10.1046/j.1365-3024.2003.00643.x. Parasite Immunol. 2003. PMID: 14521577 Review. No abstract available.
Cited by
-
Antibodies Against the Plasmodium vivax Apical Membrane Antigen 1 From the Belem Strain Share Common Epitopes Among Other Worldwide Variants.Front Cell Infect Microbiol. 2021 Mar 16;11:616230. doi: 10.3389/fcimb.2021.616230. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33796476 Free PMC article.
-
Population genetic structure of domain I of apical membrane antigen-1 in Plasmodium falciparum isolates from Hazara division of Pakistan.Malar J. 2018 Oct 26;17(1):389. doi: 10.1186/s12936-018-2539-3. Malar J. 2018. PMID: 30367656 Free PMC article.
-
Structure guided mimicry of an essential P. falciparum receptor-ligand complex enhances cross neutralizing antibodies.Nat Commun. 2023 Sep 21;14(1):5879. doi: 10.1038/s41467-023-41636-5. Nat Commun. 2023. PMID: 37735574 Free PMC article.
-
Mechanistic understanding of the aspect ratio-dependent adjuvanticity of engineered aluminum oxyhydroxide nanorods in prophylactic vaccines.Nano Today. 2022 Apr;43:101445. doi: 10.1016/j.nantod.2022.101445. Epub 2022 Mar 4. Nano Today. 2022. PMID: 35261619 Free PMC article.
-
Unraveling Haplotype Diversity of the Apical Membrane Antigen-1 Gene in Plasmodium falciparum Populations in Thailand.Korean J Parasitol. 2018 Apr;56(2):153-165. doi: 10.3347/kjp.2018.56.2.153. Epub 2018 Apr 30. Korean J Parasitol. 2018. PMID: 29742870 Free PMC article.
References
-
- WHO. World Malaria Report 2015. Geneva: World Health Organization; 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

