Volume expanders for the prevention of ovarian hyperstimulation syndrome
- PMID: 27577848
- PMCID: PMC9243766
- DOI: 10.1002/14651858.CD001302.pub3
Volume expanders for the prevention of ovarian hyperstimulation syndrome
Abstract
Background: Ovarian hyperstimulation syndrome (OHSS) is a serious and potentially fatal complication of ovarian stimulation which affects 1% to 14% of all in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles. A number of clinical studies with conflicting results have reported on the use of plasma expanders such as albumin, hydroxyethyl starch (HES), mannitol, polygeline and dextran as a possible intervention for the prevention of OHSS. Women with very high estradiol levels, high numbers of follicles or oocytes retrieved, and women with polycystic ovary syndrome (PCOS), are at particularly high risk of developing OHSS. Plasma expanders are not commonly used nowadays in ovarian hyperstimulation. This is mainly because clinical evidence on their effectiveness remains sparse, because of the low incidence of moderate and severe ovarian hyperstimulation syndrome (OHSS) and the simultaneous introduction of mild stimulation approaches, gonadotropin-releasing hormone (GnRH) antagonist protocols and the freeze-all strategy for the prevention of OHSS.
Objectives: To review the effectiveness and safety of administration of volume expanders for the prevention of moderate and severe ovarian hyperstimulation syndrome (OHSS) in high-risk women undergoing IVF or ICSI treatment cycles.
Search methods: We searched databases including the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and trial registers to September 2015; no date restrictions were used as new comparators were included in this search. The references of relevant publications were also searched. We attempted to contact authors to provide or clarify data that were unclear from trial or abstract reports.
Selection criteria: We included randomised controlled trials (RCTs) comparing volume expanders versus placebo or no treatment for the prevention of OHSS in high-risk women undergoing ovarian hyperstimulation as part of any assisted reproductive technique.
Data collection and analysis: Two review authors independently selected the studies, assessed risk of bias and extracted relevant data. The primary review outcome was moderate or severe OHSS. Other outcomes were live birth, pregnancy and adverse events. We combined data to calculate pooled Peto odds ratios (ORs) and 95% confidence intervals (CIs) for each intervention. Statistical heterogeneity was assessed using the I(2) statistic. We assessed the overall quality of the evidence for each comparison, using GRADE methods.
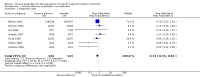
Main results: We included nine RCTs (1867 women) comparing human albumin (seven RCTs) or HES (two RCTs) or mannitol (one RCT) versus placebo or no treatment for prevention of OHSS. The evidence was very low to moderate quality for all comparisons. The main limitations were imprecision, poor reporting of study methods, and failure to blind outcome assessment.There was evidence of a beneficial effect of intravenous albumin on OHSS, though heterogeneity was substantial (Peto OR 0.67 95% CI 0.47 to 0.95, seven studies, 1452 high risk women; I² = 69%, very low quality evidence) . This suggests that if the rate of moderate or severe OHSS with no treatment is 12%, it will be about 9% (6% to12%) with the use of intravenous albumin. However, there was evidence of a detrimental effect on pregnancy rates (Peto OR 0.72 95% CI 0.55 to 0.94, I² = 42%, seven studies 1069 high risk women, moderate quality evidence). This suggests that if the chance of pregnancy is 40% without treatment, it will be about 32% (27% to 38%) with the use of albumin.There was evidence of a beneficial effect of HES on OHSS (Peto OR 0.27 95% CI 0.12 to 0.59, I² = 0%, two studies, 272 women, very low quality evidence). This suggests that if the rate of moderate or severe OHSS with no treatment is 16%, it will be about 5% (2% to 10%) with the use of HES. There was no evidence of an effect on pregnancy rates (Peto OR 1.20 95% CI 0.49 to 2.93, one study, 168 women, very low quality evidence).There was evidence of a beneficial effect of mannitol on OHSS (Peto OR 0.38, 95% CI 0.22 to 0.64, one study, 226 women with PCOS, low quality evidence). This means that if the risk of moderate or severe OHSS with no treatment is 52%, it will be about 29% (19% to 41%) with mannitol. There was no evidence of an effect on pregnancy rates (Peto OR 0.85 95% CI 0.47 to 1.55; one study, 226 women, low quality evidence).Live birth rates were not reported in any of the studies. Adverse events appeared to be uncommon, but were too poorly reported to reach any firm conclusions.
Authors' conclusions: Evidence suggests that the plasma expanders assessed in this review (human albumin, HES and mannitol) reduce rates of moderate and severe OHSS in women at high risk. Adverse events appear to be uncommon, but were too poorly reported to reach any firm conclusions, and there were no data on live birth. However, there was evidence that human albumin reduces pregnancy rates. While there was no evidence that HES, or mannitol had any influence on pregnancy rates, the evidence of effectiveness was based on very few trials which need to be confirmed in additional, larger randomised controlled trials (RCTs) before they should be considered for routine use in clinical practice.
Conflict of interest statement
None known.
Figures
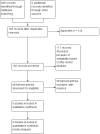













Update of
-
Intra-venous fluids for the prevention of severe ovarian hyperstimulation syndrome.Cochrane Database Syst Rev. 2011 Feb 16;(2):CD001302. doi: 10.1002/14651858.CD001302.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Aug 31;(8):CD001302. doi: 10.1002/14651858.CD001302.pub3. PMID: 21328249 Updated.
References
References to studies included in this review
Bellver 2003 {published data only}
-
- Bellver J, Munoz EA, Ballesteros A, Soares SR, Bosch E, Simon C, et al. Intravenous albumin does not prevent moderate–severe ovarian hyperstimulation syndrome in high‐risk IVF patients: a randomized controlled study. Human Reproduction 2003;18(11):2283–8. - PubMed
Gokmen 2001 {published data only}
-
- Gokmen O, Ugur M, Ekin M, Keles G, Turan C, Oral H. Intravenous albumin versus hydroxyethyl starch for the prevention of ovarian hyperstimulation in an in‐vitro fertilization programme: a prospective randomised placebo controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;96(2):187‐92. - PubMed
Isik 1996 {published data only}
-
- Isik AZ, Gokmen O, Zeyneloglu HB, Kara S, Keles G, Gulekli B. Intravenous albumin prevents moderate‐severe ovarian hyperstimulation in in vitro fertilisation patients: a prospective randomized and controlled study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;70:170‐83. - PubMed
Isikoglu 2007 {published data only}
-
- Isikoglu M, Berkkanoglu M, Senturk Z, Ozgur K. Human albumin does not prevent ovarian hyperstimulation syndrome in assisted reproductive technology program: a prospective randomized placebo‐controlled double blind study. Fertility and Sterility 2007;88(4):982‐5. - PubMed
Koike 1999 {published data only}
-
- Koike E, Araki S, Ogawa S, Minakami H, Sato I. Does i.v albumin prevent ovarian hyperstimulation syndrome?. Human Reproduction 1999;14(7):1920. - PubMed
Konig 1998 {published data only}
-
- Konig E, Bussen S, Sütterlin M, Steck T. Prophylactic intravenous hydroxyethyle starch solution prevents moderate‐severe ovarian hyperstimulation in in‐vitro fertilization patients: a prospective, randomized, double‐blind and placebo‐controlled study. Human Reproduction 1998;13(9):2421‐4. - PubMed
Saremi 2003 {published and unpublished data}
-
- Saremi A, Alam M, Motaghi M. Administration of mannitol to prevent severe ovarian hyperstimulation syndrome: A randomized controlled trial. Middle East Fertility Society Journal 2003;8(2):159‐163.
Shalev 1995 {published data only}
-
- Shalev E, Giladi Y, Matilsky M, Ben‐Ami M. Decreased incidence of severe ovarian hyperstimulation syndrome in high risk in‐vitro fertilisation patients receiving intravenous albumin: a prospective study. Human Reproduction 1995;10:1373‐6. - PubMed
Shoham 1994 {published data only}
-
- Shoham Z, Weissman A, Barash A, Borenstein R, Schachter M, Insler V. Intravenous albumin for the prevention of severe ovarian hyperstimulation syndrome in an invitro fertilisation program: a prospective, randomised, placebo‐controlled study. Fertility and Sterility 1994;62:137‐42. - PubMed
References to studies excluded from this review
Abramov 2001 {published data only}
-
- Abramov Y, Fatum M, Abrahamove D, Schenker JC. Hydroxyethyl starch versus human albumin for the treatment of severe ovarian hyperstimulation syndrome: a preliminary report. Fertility and Sterility 2001;75:1228‐30. - PubMed
-
- Abramov Y, Fatum M, Fasouliotis S, Abrahamov D, Schenker JC. Hydroxyethylstarch versus human albumin for the treatment of severe ovarian hyperstimulation syndrome. Fertility and Sterility. Abstracts of the 17th Annual meeting of the ESHRE 2001;16(1):121. - PubMed
Abuzeid 2010 {published data only}
-
- Abuzeid M, Abed S, Salem W, Joseph S, Ashraf M, Rizk P.B. The effectiveness of 6% hydroxyethyle starch solution in reducing the incidence of severe hyperstimulation syndrome in polycystic ovarian disease patients: A prospective cohort clinical trial. Journal of Reproductive Medicine and Endocrinology, IFFS 2010 September 12‐16 Munich;7(4):240‐383.
Ahmadi 2010 {published data only}
-
- Ahmadi Sh, Rahmani E, Oskouian H. Cabergoline versus human albumin in prophylaxis ovarian hyperstimulation syndrome. Reproductive BioMedicine Online 2010;Suppl. 3:S41–S42.
Asch 1993 {published data only}
-
- Asch R, Ivery G, Goldsman M. The use of intravenous albumin in patients at high risk for severe ovarian hyperstimulation syndrome. Human Reproduction 1993;8:1015‐20. - PubMed
Ben‐Chetrit 2001 {published data only}
-
- Ben Chetrit A, Eldar‐Geva T, Gal M, Huerta M, Mimon T, Algur N, et al. The questionable use of albumin for the prevention of ovarian hyperstimulation syndrome in an IVF programme: a randomized placebo‐controlled trial. Human Reproduction 2001;16(9):1880‐4. - PubMed
Ben‐Rafael 1995 {published data only}
-
- Ben‐Rafael Z, Orvieto R, Dekel A, Peleg D, Gruzman C. Intravenous albumin and the prevention of severe ovarian hyperstimulation syndrome. Human Reproduction 1995;10(10):2750‐2. - PubMed
Cambiaghi 2005 {published data only}
-
- Cambiaghi AS, Castellotti DS. Oral whey protein for preventing ovarian hyperstimulation syndrome. Fertility and Sterility. Abstracts from the annual meeting of ASRM 84 Suppl 1;84(1):315.
Chen 1997 {published data only}
-
- Chen C, Chen S, Wu M, Ho H, Yang J, Yang Y. Intravenous albumin does not prevent the development of severe ovarian hyperstimulation syndrome. Fertility and Sterility 1997;68:287‐91. - PubMed
Chen 2003 {published data only}
-
- Chen CD, Chao KH, Yang JH, Chen SU, Ho HN, Yang YS. Comparison of coasting and intravenous albumin in the prevention of ovarian hyperstimulation syndrome. Fertility and Sterility 2003;80(1):86‐90. - PubMed
Costabile 2000 {published data only}
-
- Costabile L, Unfer V, Manna C, Gerli S, Rossetti D, Renzo GC. Use of intramuscular progesterone versus intravenous albumin for the prevention of ovarian hyperstimulation syndrome. Gynecologic and Obstetric Investigation 2000;50(3):182‐5. - PubMed
Dmitrieva 2010 {published data only}
-
- Dmitrieva NV, Yakovenko SA, Zorina IV, Voznesenskaya YV, Apryshko VP. Comparison of calcium gluconate and starch solution in the treatment of ovarian hyperstimulation syndrome. Reproductive BioMedicine Online 2010;3:S41–S42.
Egbase 1997 {published data only}
-
- Egbase PE, Makhseed M, Al Sharhan M, Grdzinskas JG. Timed unilateral ovarian follicular aspiration prior to administration of human chorionic gonadotrophin for the prevention of severe ovarian hyperstimulation syndrome in invitro fertilisation: a prospective randomised study. Human Reproduction 1997;12:2603‐6. - PubMed
El‐Khayat 2015 {published data only}
-
- El‐Khayat W, Elsadek M. Calcium infusion for the prevention of ovarian hyperstimulation syndrome: a double‐blind randomized controlled trial. Fertility and Sterility 2015;103(1):101‐5. - PubMed
Endo 2004 {published data only}
-
- Endo T, Kitajima Y, Hayashi T, Fujii M, Hata H, Azumaguchi A. Low‐molecular‐weight dextran infusion is more effective for the treatment of hemoconcentration due to severe ovarian hyperstimulation syndrome than human albumin infusion. Fertility and Sterility 2004;82(5):1449‐51. - PubMed
Fan 2006 {published data only}
-
- Fan YH, Qiao J, Chen XN, Wang HY, Ma CH, Liu P. Assisted reproductive Intravenous hydroxyethyl starch versus human albumin for prevention of the ovarian hyperstimulation syndrome. VIII FIGO World Congress of Gynecology and Obstetrics 2006;2:34.
-
- Fan YH, Qiao J, Chen XN, Wang HY, Ma CH, Liu P. Intravenous hydroxyethyl starch versus human albumin for prevention of the ovarian hyperstimulation syndrome. Fertility and Sterility 2006;86 Suppl 2:136.
Fluker 2000 {published data only}
-
- Fluker MR, Copeland JE, Yuzpe AA. An ounce of prevention: outpatient management of the ovarian hyperstimulation syndrome. Fertility and Sterility 2000;73(4):821‐4. - PubMed
Gamzu 2002 {published data only}
-
- Gamzu R, Almog B, Levin Y, Avni A, Lessing BJ, Baram A. Comparative efficacy of hydroxyethyl starch and Haemaccel for the treatment of severe ovarian hyperstimulation syndrome. Fertility and Sterility 2002;77(6):1302‐3. - PubMed
Gokmen 2005 {published data only}
-
- Gokmen O, Ozcan S, Erman Akar M, Ugur M. A randomized prospective placebo‐controlled study of intravenous albumin vs. hydroxyethyl starch for the prevention of ovarian hyperstimulation in an in‐vitro fertilization programme. Fertility and Sterility: Abstracts of ASRM 2005;84(Suppl 1):308.
Graf 1997 {published data only}
-
- Graf AM, Fischer R, Naether ONJ, Baukloh V, Tafel J, Nuckel M. Reduced incidence of ovarian hyperstimulation syndrome by prophylactic infusion of hydroxyethyl starch solution in an in‐vitro fertilization programme. Human Reproduction 1997;12(12):2599‐602. - PubMed
Halme 1995 {published data only}
-
- Halme J, Toma SK, Talbert LM. A case of severe ovarian hyperstimulation in a healthy oocyte donor. Fertility and Sterility 1995;64(4):857‐9. - PubMed
Isik 1997 {published data only}
-
- Isik AZ, Ozgun OD, Kahraman S, Polat G, Vicdan K, Biberoglu K, et al. Intravenous albumin combined with low dose human chorionic gonadotrophin and late step‐down administration of menotropins are effective in prevention of severe ovarian hyperstimulation syndrome in high‐risk patients in an in vitro fertilisation program. Middle East Fertility Society Journal 1997;2/3:238‐42.
Isik 2001 {published data only}
-
- Isik AZ, Vicdan K. Combined approach as an effective method in the prevention of severe ovarian hyperstimulation syndrome. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;97(2):208‐12. - PubMed
Kissler 2001 {published data only}
-
- Kissler S, Neidhardt B, Siebzehnrübl E, Schmitt H, Tschaikowsky K, Wildt L. The detrimental role of colloidal volume substitutes in severe ovarian hyperstimulation syndrome: a case report. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;99(1):131‐4. - PubMed
Koike 2000 {published data only}
-
- Koike E, Araki S, Ogawa S, Minakami H, Sayama H, Shibahara H, et al. Clinical efficacy of peritoneovenous shunting for the treatment of sever ovarian hyperstimulation syndrome. Human Reproduction 2000;15(1):113‐7. - PubMed
Kumbak 2005 {published data only}
-
- Kumbak B, Kahraman S, Karlikaya G, Karagozoglu H, Sen T, Hatirnaz S. Intravenous albumin prophylaxis in high risk patients for moderate to severe ovarian hyperstimulation syndrome. Fertility and Sterility 2005;84 Suppl(1):264.
Lewit 1996 {published data only}
-
- Lewit N, Kol S, Ronen N, Itskovitz‐Eldor J. Does intravenous administration of human albumin prevent severe ovarian hyperstimulation syndrome?. Fertility and Sterility 1996;66(4):654‐6. - PubMed
Lincolin 2002 {published data only}
Matorras 2013 {published and unpublished data}
-
- Matorras R, Andrés M, Mendoza R, Prieto B, Pijoan JI, Expósito A. Prevention of ovarian hyperstimulation syndrome in GnRH agonist IVF cycles in moderate risk patients: randomized study comparing hydroxyethyl starch versus cabergoline and hydroxyethyl starch. European Journal of Obstetrics Gynecology and Reproductive Biology 2013;170(2):439‐43. - PubMed
Milacic 1996 {published data only}
-
- Milacic D, Radonjic G, Matijasevic S, Kontic O. Prevention of ovarian hyperstimulation syndrome with iv albumin. Human Reproduction 1996;11(1):216.
Mukherjee 1995 {published data only}
-
- Mukherjee T, Copperman AB, Sandler B, Bustillo M, Grunfeld L. Severe ovarian hyperstimulation despite prophylactic albumin at the time of oocyte retrieval for in vitro fertilization and embryo transfer. Fertility and Sterility 1995;64(3):641‐3. - PubMed
Naredi 2013 {published and unpublished data}
Ndukwe 1997 {published data only}
-
- Ndukwe G, Thornton S, Fishel S, Dowell K, Aloum M. Severe ovarian hyperstimulation syndrome: is it really preventable by prophylactic intravenous albumin?. Fertility and Sterility 1997;68(5):851‐4. - PubMed
Ng 1995 {published data only}
-
- Ng E, Leader A, Claman P, Domingo M, Spence JEH. Intravenous albumin does not prevent the development of severe ovarian hyperstimulation syndrome in an in‐vitro fertilization programme. Human Reproduction 1995;10(4):807‐10. - PubMed
Panay 1999 {published data only}
-
- Iammarone E, Panay N, ZosmerA, TozerA, Hussain S, Wilson C, et al. Does the prophylactic use of intravenous albumin prevent ovarian hyperstimulation syndrome? A randomised prospective study. Human Fertility 1999;2(2):179‐180.
-
- Panay NA, Iammorrone E, Zosmer A, Tozer A, Hussain S. Does the prophylactic use of intravenous albumin prevent ovarian hyperstimulation syndrome? A randomized prospective study. Abstracts from the 15th Annual Meeting of the ESHRE, Tours, France 1999;14:105.
Shaker 1996 {published data only}
-
- Shaker AG, Zosmer A, Dean N, Berik JS, Jacob HS, Tan SL. Comparison of intravenous albumin and transfer of fresh embryos with cryopreservation of all embryos for subsequent transfer in prevention of ovarian of ovarian hyperstimulation syndrome. Fertility and Sterility 1996;65:992‐6. - PubMed
Tan 1992 {published data only}
-
- Tan Sl, Balen A, Hussein E, Campbell S, Jacobs HS. The administration of glucocorticoids for the prevention of ovarian hyperstimulation syndrome in invitro fertilisation: a prospective randomised study. Fertility and Sterility 1992;58:378‐83. - PubMed
Tehraninejad 2012 {published and unpublished data}
Torabizadeh 2013 {published and unpublished data}
Yakovenko 2009 {published and unpublished data}
-
- Yakovenko SA, Zorina IV, Dmitrieva NV, Troshina MN, Apryshko VP, Voznesenskaya JV. Intravenous administration of calcium in ovarian hyperstimulation syndrome (OHSS) prevention. European Journal of Obstetrics Gynecology and Reproductive Biology 2011;1:s20.
Additional references
Aboulghar 2003
-
- Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Human Reproduction Update 2003;9(3):275‐89. - PubMed
Cerrilo 2009
-
- Cerrillo M, Pacheco A, Rodríguez S, Mayoral M, Ruiz M, García Velasco JA. Differential regulation of VEGF, Cadherin, and Angiopietin 2 by trigger oocyte maturation with GnRHa vs hCG in donors: try to explain the lower OHSS incidence. Abstracts of the 25th annual meeting of the ESHRE 2009;24(1):i60.
Chen 2003a
-
- Chen D, Burmeister L, Goldschlag D, Rosenwaks Z. Ovarian hyperstimulation syndrome: strategies for prevention. Reproductive Biomedicine Online 2003;7(1):43‐9. - PubMed
D'Angelo 2007
D'Angelo 2011
Delvigne 2002
-
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Human Reproduction Update 2002;8:559‐77. - PubMed
Edwards 2007
-
- Edwards RG. IVF, IVM, natural cycle IVF, minimal stimulation IVF ‐ time for a rethink. Reproductive Biomedicine Online 2007;15:106‐19. - PubMed
Egbase 2000
-
- Egbase PE. Severe OHSS: how many cases are preventable?. Human Reproduction 2000;15:8‐10. - PubMed
Engmann 2008
-
- Engmann L, DiLugie A, Schmidt D, Nulsen J, Maier D, Benavida C. The use of gonadotropin‐releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high‐risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomised controlled study. Fertility and Sterility 2008;89(1):84‐91. - PubMed
Enskog 2001
-
- Enskog A, Nilsson L, Brännström M. Plasma levels of free vascular endothelial growth factor(165) (VEGF(165)) are not elevated during gonadotropin stimulation in in vitro fertilization (IVF) patients developing ovarian hyperstimulation syndrome (OHSS): results of a prospective cohort study with matched controls. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;96:196‐201. - PubMed
Foong 2006
-
- Foong LC, Bhagavath BA, Kumar J, Ng SC. Ovarian hyperstimulation syndrome is associated with reversible impairment of vascular reactivity. Fertility and Sterility 2002;78(6):1159‐63. - PubMed
Garcia‐Velasco 2003
-
- Garcia‐Velasco JA, Pellicer A. New concepts in the understanding of the ovarian hyperstimulation syndrome. Current Opinion in Obstetrics and Gynecology 2003;15(3):251‐6. - PubMed
Golan 1989
-
- Golan A, Ron‐el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstetrical & Gynecological Survey 1989;44(6):430‐40. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011; Vol. Available from www.cochrane‐handbook.org..
Humaidan 2010
-
- Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertility and Sterility 2010;94(2):389–400. - PubMed
Jee 2010
-
- Jee BC, Suh CS, Kim YB, Kim SH, Choi YM, Kim JG, et al. Administration of intravenous albumin around the time of oocyte retrieval reduces pregnancy rate without preventing ovarian hyperstimulation syndrome: a systematic review and meta‐analysis. Gynecologic and Obstetric Investigation 2010;70(1):47‐54. Epub 2010 Feb 19. - PubMed
Kol 2008
Loutradis 2006
-
- Loutradis D, Kiapekou E, Zapanti E, Antsaklis A. Oocyte maturation in assisted reproductive techniques. Annals of the New York Academy of Sciences 2006;1092:235‐46. - PubMed
Mathur 2000
-
- Mathur RS, Jenkins JM. Is ovarian hyperstimulation syndrome associated with a poor obstetric outcome?. BJOG 2000;107:943‐6. - PubMed
Navot 1988
-
- Navot D, Relou A, Birkenfeld A, Rabinowitz R, Brzezinski A, Margalioth EJ. Risk factors and prognostic variables in the ovarian hyperstimulation syndrome. American Journal of Obstetrics and Gynecology 1988;159:210‐5. - PubMed
Navot 1992
-
- Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies:prevention and treatment. Fertility & Sterility 1992;58:249‐61. - PubMed
Rizk 1997
-
- Rizk B, Aboulghar M, Smitz J, Ron‐El R. The role of vascular endothelial growth factor and interleukins in the pathogenesis of severe ovarian hyperstimulation syndrome. Human Reproduction Update 1997;3:255‐6. - PubMed
Rizk 1999
-
- Rizk B, Aboulghar MA. Classification, pathophysiology and management of ovarian hyperstimulation syndrome. In: Brinsden P editor(s). In‐Vitro Fertilization and Assisted Reproduction. New York, London: The Parthenon Publishing Group, 1999:131‐55.
Schenker 1978
-
- Schenker JG, Weinstein D. Ovarian hyperstimulation syndrome: a current survey. Fertility and Sterility 1978;30:225‐68. - PubMed
Shawkat 2012
-
- Shawkat H, Westwood M, Mortimer A. Mannitol: a review of its clinical uses. Continuing Education in Anaesthesia, Critical Care & Pain 2012;12(2):82‐5.
Tang 2012
Venetis 2011
-
- Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Papadimas I, Tarlatzis BC. Intravenous albumin administration for the prevention of severe ovarian hyperstimulation syndrome: a systematic review and metaanalysis. Fertility and Sterilility 2011;95(1):188‐96. - PubMed
Vloeberghs 2009
-
- Vloeberghs V, Peeraer K, Pexsters A, D’Hooghe T. Ovarianhyperstimulation syndrome and complications of ART. Best Practice & Research. Clinical Obstetrics & Gynaecology 2009;23(5):691‐709. - PubMed
WHO 1973
-
- WHO. Agents stimulating gonadal function in human. World Health Organization Technical Report Series 1973:514‐20. - PubMed
Yan 1993
-
- Yan Z, Weich HA, Bernart W, Breckwoldt M, Neulen J. Ascular endothelial growth factor (VEGF) messenger ribonucleic acid (mRNA) expression in luteinized human granulosa cells in vitro. Journal of Clinical Endocrinology and Metabolism 1993;77:1723‐5. - PubMed
Youssef 2014
-
- Youssef MAFM, Veen F, Al‐Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, et al. Gonadotropin‐releasing hormone agonist versus HCG for oocyte triggering in antagonist‐assisted reproductive technology. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD008046.pub4] - DOI - PMC - PubMed
References to other published versions of this review
Youssef 2002
-
- Youssef MA, Al‐Inany HG, Evers JHANSLH, Aboulghar M. Intra‐venous fluids preventing severe ovarian hyperstimulation syndrome. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001302] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

