Adenosine arrests breast cancer cell motility by A3 receptor stimulation
- PMID: 27577957
- PMCID: PMC5124008
- DOI: 10.1007/s11302-016-9531-6
Adenosine arrests breast cancer cell motility by A3 receptor stimulation
Abstract
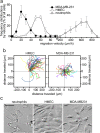
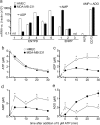
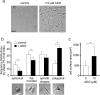
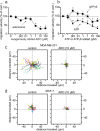
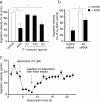
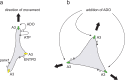
In neutrophils, adenosine triphosphate (ATP) release and autocrine purinergic signaling regulate coordinated cell motility during chemotaxis. Here, we studied whether similar mechanisms regulate the motility of breast cancer cells. While neutrophils and benign human mammary epithelial cells (HMEC) form a single leading edge, MDA-MB-231 breast cancer cells possess multiple leading edges enriched with A3 adenosine receptors. Compared to HMEC, MDA-MB-231 cells overexpress the ectonucleotidases ENPP1 and CD73, which convert extracellular ATP released by the cells to adenosine that stimulates A3 receptors and promotes cell migration with frequent directional changes. However, exogenous adenosine added to breast cancer cells or the A3 receptor agonist IB-MECA dose-dependently arrested cell motility by simultaneous stimulation of multiple leading edges, doubling cell surface areas and significantly reducing migration velocity by up to 75 %. We conclude that MDA-MB-231 cells, HMEC, and neutrophils differ in the purinergic signaling mechanisms that regulate their motility patterns and that the subcellular distribution of A3 adenosine receptors in MDA-MB-231 breast cancer cells contributes to dysfunctional cell motility. These findings imply that purinergic signaling mechanisms may be potential therapeutic targets to interfere with the motility of breast cancer cells in order to reduce the spread of cancer cells and the risk of metastasis.
Keywords: ATP; Adenosine; Adenosine receptor; Breast cancer; Cell motility; Purinergic signaling.
Conflict of interest statement
The authors declare that they have no conflicts of interest. Ethical approval All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent Informed consent was obtained from all individual participants included in the study.
Figures

Similar articles
-
ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors.Science. 2006 Dec 15;314(5806):1792-5. doi: 10.1126/science.1132559. Science. 2006. PMID: 17170310
-
An adenosine analog (IB-MECA) inhibits anchorage-dependent cell growth of various human breast cancer cell lines.Int J Biochem Cell Biol. 2004 Aug;36(8):1502-9. doi: 10.1016/j.biocel.2003.12.001. Int J Biochem Cell Biol. 2004. PMID: 15147729
-
Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis.Oncogene. 2015 Apr 2;34(14):1831-42. doi: 10.1038/onc.2014.113. Epub 2014 May 19. Oncogene. 2015. PMID: 24837364 Free PMC article.
-
A3 adenosine receptor as a target for cancer therapy.Anticancer Drugs. 2002 Jun;13(5):437-43. doi: 10.1097/00001813-200206000-00001. Anticancer Drugs. 2002. PMID: 12045454 Review.
-
Stimulation of adenosine A3 receptors in cerebral ischemia. Neuronal death, recovery, or both?Ann N Y Acad Sci. 1999;890:93-106. doi: 10.1111/j.1749-6632.1999.tb07984.x. Ann N Y Acad Sci. 1999. PMID: 10668416 Review.
Cited by
-
Adenosine receptors in breast cancer.Mol Biol Rep. 2024 Mar 29;51(1):464. doi: 10.1007/s11033-024-09382-z. Mol Biol Rep. 2024. PMID: 38551734 Review.
-
Morphological and Motility Features of the Stable Bleb-Driven Monopodial Form of Entamoeba and Its Importance in Encystation.Infect Immun. 2020 Jul 21;88(8):e00903-19. doi: 10.1128/IAI.00903-19. Print 2020 Jul 21. Infect Immun. 2020. PMID: 32393510 Free PMC article.
-
Role of ENPP1 in cancer pathogenesis: Mechanisms and clinical implications (Review).Oncol Lett. 2024 Oct 3;28(6):590. doi: 10.3892/ol.2024.14722. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39411204 Free PMC article. Review.
-
Drugs Targeting the A3 Adenosine Receptor: Human Clinical Study Data.Molecules. 2022 Jun 8;27(12):3680. doi: 10.3390/molecules27123680. Molecules. 2022. PMID: 35744805 Free PMC article. Review.
-
From purines to purinergic signalling: molecular functions and human diseases.Signal Transduct Target Ther. 2021 Apr 28;6(1):162. doi: 10.1038/s41392-021-00553-z. Signal Transduct Target Ther. 2021. PMID: 33907179 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

