Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis
- PMID: 27578049
- PMCID: PMC5013657
- DOI: 10.1038/ncomms12658
Neutrophil recruitment limited by high-affinity bent β2 integrin binding ligand in cis
Abstract
Neutrophils are essential for innate immunity and inflammation and many neutrophil functions are β2 integrin-dependent. Integrins can extend (E(+)) and acquire a high-affinity conformation with an 'open' headpiece (H(+)). The canonical switchblade model of integrin activation proposes that the E(+) conformation precedes H(+), and the two are believed to be structurally linked. Here we show, using high-resolution quantitative dynamic footprinting (qDF) microscopy combined with a homogenous conformation-reporter binding assay in a microfluidic device, that a substantial fraction of β2 integrins on human neutrophils acquire an unexpected E(-)H(+) conformation. E(-)H(+) β2 integrins bind intercellular adhesion molecules (ICAMs) in cis, which inhibits leukocyte adhesion in vitro and in vivo. This endogenous anti-inflammatory mechanism inhibits neutrophil aggregation, accumulation and inflammation.
Figures
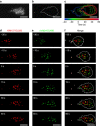

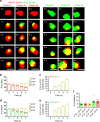
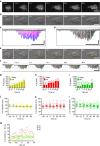
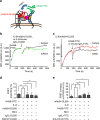


References
-
- Iversen M. B. et al.. An innate antiviral pathway acting before interferons at epithelial surfaces. Nat. Immunol. 17, 150–158 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

