Aging increases the susceptibility of hepatic inflammation, liver fibrosis and aging in response to high-fat diet in mice
- PMID: 27578257
- PMCID: PMC5061686
- DOI: 10.1007/s11357-016-9938-6
Aging increases the susceptibility of hepatic inflammation, liver fibrosis and aging in response to high-fat diet in mice
Abstract
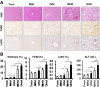
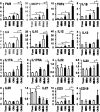
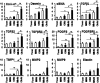
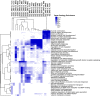
We aimed to investigate whether aging increases the susceptibility of hepatic and renal inflammation or fibrosis in response to high-fat diet (HFD) and explore the underlying genetic alterations. Middle (10 months old) and old (20 months old) aged, male C57BL/6N mice were fed either a low-fat diet (4 % fat) or HFD (60 % fat) for 4 months. Young (3 months old) aged mice were included as control group. HFD-induced liver and kidney injuries were analyzed by serum and urine assay, histologic staining, immunohistochemistry, and reverse-transcription real-time quantitative polymerase chain reaction. Total RNA sequencing with next-generation technology was done with RNA extracted from liver tissues. With HFD feeding, aged was associated with higher serum alanine aminotransferase levels, marked infiltration of hepatic macrophages, and increased expression of inflammatory cytokines (MCP1, TNF-α, IL-1β, IL-6, IL-12, IL-17A). Importantly, aged mice showed more advanced hepatic fibrosis and increased expression of fibrogenic markers (Col-I-α1, αSMA, TGF-β1, TGF-β2, TGFβRII, PDGF, PDGFRβII, TIMP1) in response to HFD. Aged mice fed on HFD also showed increased oxidative stress and TLR4 expression. In the total RNA seq and gene ontology analysis of liver, old-aged HFD group showed significant up-regulation of genes linked to innate immune response, immune response, defense response, inflammatory response compared to middle-aged HFD group. Meanwhile, aging and HFD feeding showed significant increase in glomerular size and mesangial area, higher urine albumin/creatinine ratio, and advanced renal inflammation or fibrosis. However, the difference of HFD-induced renal injury between old-aged group and middle-aged group was not significant. The susceptibility of hepatic fibrosis as well as hepatic inflammation in response to HFD was significantly increased with aging. In addition, aging was associated with glomerular alterations and increased renal inflammation or fibrosis, while the differential effect of aging on HFD-induced renal injury was not remarkable as shown in the liver.
Keywords: Aging; Fibrosis; High-fat diet; Liver.
Conflict of interest statement
None. Financial support and sponsorship This research was supported by The GlaxoSmithKline Research Fund of the Korean Association for the study of the Liver (In Hee Kim). Support: NIH R01MH094151-01 and MH019934, and the Sam and Rose Stein Institute for Research on Aging, University of California, San Diego.
Figures


References
-
- Organization WH . Global health and aging: World Health Organization. 2011.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
