Bacterial Cell Growth Inhibitors Targeting Undecaprenyl Diphosphate Synthase and Undecaprenyl Diphosphate Phosphatase
- PMID: 27578312
- PMCID: PMC5155509
- DOI: 10.1002/cmdc.201600342
Bacterial Cell Growth Inhibitors Targeting Undecaprenyl Diphosphate Synthase and Undecaprenyl Diphosphate Phosphatase
Abstract
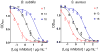
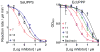
We synthesized a series of benzoic acids and phenylphosphonic acids and investigated their effects on the growth of Staphylococcus aureus and Bacillus subtilis. One of the most active compounds, 5-fluoro-2-(3-(octyloxy)benzamido)benzoic acid (7, ED50 ∼0.15 μg mL-1 ) acted synergistically with seven antibiotics known to target bacterial cell-wall biosynthesis (a fractional inhibitory concentration index (FICI) of ∼0.35, on average) but had indifferent effects in combinations with six non-cell-wall biosynthesis inhibitors (average FICI∼1.45). The most active compounds were found to inhibit two enzymes involved in isoprenoid/bacterial cell-wall biosynthesis: undecaprenyl diphosphate synthase (UPPS) and undecaprenyl diphosphate phosphatase (UPPP), but not farnesyl diphosphate synthase, and there were good correlations between bacterial cell growth inhibition, UPPS inhibition, and UPPP inhibition.
Keywords: Staphylococcus aureus; benzoic acids; cell-wall biosynthesis; drug discovery; membrane proteins.
© 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

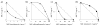
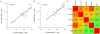
Similar articles
-
Discovery and Characterization of a Class of Pyrazole Inhibitors of Bacterial Undecaprenyl Pyrophosphate Synthase.J Med Chem. 2016 Aug 11;59(15):7299-304. doi: 10.1021/acs.jmedchem.6b00746. Epub 2016 Jul 20. J Med Chem. 2016. PMID: 27379833
-
Structure, Function, and Inhibition of Staphylococcus aureus Heptaprenyl Diphosphate Synthase.ChemMedChem. 2016 Sep 6;11(17):1915-23. doi: 10.1002/cmdc.201600311. Epub 2016 Jul 26. ChemMedChem. 2016. PMID: 27457559 Free PMC article.
-
Isoprenoid Biosynthesis Inhibitors Targeting Bacterial Cell Growth.ChemMedChem. 2016 Oct 6;11(19):2205-2215. doi: 10.1002/cmdc.201600343. Epub 2016 Aug 30. ChemMedChem. 2016. PMID: 27571880 Free PMC article.
-
Undecaprenyl diphosphate synthase, a cis-prenyltransferase synthesizing lipid carrier for bacterial cell wall biosynthesis.Mol Membr Biol. 2012 Nov;29(7):267-73. doi: 10.3109/09687688.2012.674162. Epub 2012 Apr 4. Mol Membr Biol. 2012. PMID: 22471620 Review.
-
Recent Advances in the Development of Undecaprenyl Pyrophosphate Synthase Inhibitors as Potential Antibacterials.Curr Med Chem. 2016;23(5):464-82. doi: 10.2174/0929867323666151231094854. Curr Med Chem. 2016. PMID: 26718796 Review.
Cited by
-
Investigations into the Antibacterial Mechanism of Action of Viridicatumtoxins.ACS Infect Dis. 2020 Jul 10;6(7):1759-1769. doi: 10.1021/acsinfecdis.0c00031. Epub 2020 Jun 4. ACS Infect Dis. 2020. PMID: 32437130 Free PMC article.
-
New aspects of microbial vitamin K2 production by expanding the product spectrum.Microb Cell Fact. 2021 Apr 13;20(1):84. doi: 10.1186/s12934-021-01574-7. Microb Cell Fact. 2021. PMID: 33849534 Free PMC article. Review.
-
Crystal structure of undecaprenyl-pyrophosphate phosphatase and its role in peptidoglycan biosynthesis.Nat Commun. 2018 Mar 14;9(1):1078. doi: 10.1038/s41467-018-03477-5. Nat Commun. 2018. PMID: 29540682 Free PMC article.
-
Anthranilic Acid Inhibitors of Undecaprenyl Pyrophosphate Synthase (UppS), an Essential Enzyme for Bacterial Cell Wall Biosynthesis.Front Microbiol. 2019 Jan 14;9:3322. doi: 10.3389/fmicb.2018.03322. eCollection 2018. Front Microbiol. 2019. PMID: 30692977 Free PMC article.
-
Optimization of oil yield of Pelargonium graveolens L'Hér using Box-Behnken design in relation to its antimicrobial activity and in silico study.Sci Rep. 2023 Nov 14;13(1):19887. doi: 10.1038/s41598-023-47170-0. Sci Rep. 2023. PMID: 37963988 Free PMC article.
References
-
- Antibiotic resistance threats in the United States. Executive Summary. 2013 http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
-
- Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. Science. 1999;285:1573–1576. - PubMed
-
- Peukert S, Sun Y, Zhang R, Hurley B, Sabio M, Shen X, Gray C, Dzink-Fox J, Tao J, Cebula R, Wattanasin SM. Bioorg Med Chem Lett. 2008;18:1840–1844. - PubMed
- Zhu W, Zhang Y, Sinko W, Hensler ME, Olson J, Molohon KJ, Lindert S, Cao R, Li K, Wang KM. Proc Natl Acad Sci USA. 2013;110:123–128. - PMC - PubMed
- Czarny TL, Brown ED. ACS Infectious Diseases. 2016 doi: 10.1021/acsinfecdis.6b00044. - DOI - PubMed
-
- Jahnke W, Rondeau JM, Cotesta S, Marzinzik A, Pelle X, Geiser M, Strauss A, Gotte M, Bitsch F, Hemmig R, Henry C, Lehmann S, Glickman JF, Roddy TP, Stout SJ, Grenn JR. Nat Chem Biol. 2010;6:660–666. - PubMed
-
- Eliopoulos GM, Moellering RC. In: Antibiotics in Laboratory Medicine. Lorian V, editor. Williams & Wilkins Publishing Co; 1998. pp. 330–396.
- Singh PK, Tack BF, McCray PB, Welsh MJM. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

