Identification, visualization and clonal analysis of intestinal stem cells in fish
- PMID: 27578784
- PMCID: PMC5087619
- DOI: 10.1242/dev.134098
Identification, visualization and clonal analysis of intestinal stem cells in fish
Abstract
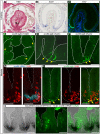
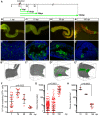
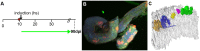
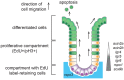
Recently, a stochastic model of symmetrical stem cell division followed by neutral drift has been proposed for intestinal stem cells (ISCs), which has been suggested to represent the predominant mode of stem cell progression in mammals. In contrast, stem cells in the retina of teleost fish show an asymmetric division mode. To address whether the mode of stem cell division follows phylogenetic or ontogenetic routes, we analysed the entire gastrointestinal tract of the teleost medaka (Oryzias latipes). X-ray microcomputed tomography shows a correlation of 3D topography with the functional domains. Analysis of ISCs in proliferation assays and via genetically encoded lineage tracing highlights a stem cell niche in the furrow between the long intestinal folds that is functionally equivalent to mammalian intestinal crypts. Stem cells in this compartment are characterized by the expression of homologs of mammalian ISC markers - sox9, axin2 and lgr5 - emphasizing the evolutionary conservation of the Wnt pathway components in the stem cell niche of the intestine. The stochastic, sparse initial labelling of ISCs ultimately resulted in extended labelled or unlabelled domains originating from single stem cells in the furrow niche, contributing to both homeostasis and growth. Thus, different modes of stem cell division co-evolved within one organism, and in the absence of physical isolation in crypts, ISCs contribute to homeostatic growth.
Keywords: Cell division mode; Digestive tract; Intestinal stem cells; Medaka.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Aghaallaei N., Gruhl F., Schaefer C. Q., Wernet T., Weinhardt V., Centanin L., Loosli F., Baumbach T. and Wittbrodt J. (2016). Data from: Identification, visualization and clonal analysis of intestinal stem cells in fish. Dryad Digital Repository. doi:10.5061/dryad.591gf 10.5061/dryad.591gf - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

