Targeting T1 and T2 dual modality enhanced magnetic resonance imaging of tumor vascular endothelial cells based on peptides-conjugated manganese ferrite nanomicelles
- PMID: 27578974
- PMCID: PMC4998025
- DOI: 10.2147/IJN.S104686
Targeting T1 and T2 dual modality enhanced magnetic resonance imaging of tumor vascular endothelial cells based on peptides-conjugated manganese ferrite nanomicelles
Abstract
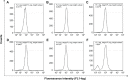
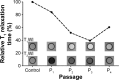
Tumor angiogenesis plays very important roles for tumorigenesis, tumor development, metastasis, and prognosis. Targeting T1/T2 dual modality magnetic resonance (MR) imaging of the tumor vascular endothelial cells (TVECs) with MR molecular probes can greatly improve diagnostic sensitivity and specificity, as well as helping to make an early diagnosis of tumor at the preclinical stage. In this study, a new T1 and T2 dual modality nanoprobe was successfully fabricated. The prepared nanoprobe comprise peptides CL 1555, poly(ε-caprolactone)-block-poly(ethylene glycol) amphiphilic copolymer shell, and dozens of manganese ferrite (MnFe2O4) nanoparticle core. The results showed that the hydrophobic MnFe2O4 nanoparticles were of uniform spheroidal appearance and narrow size distribution. Due to the self-assembled nanomicelles structure, the prepared probes were of high relaxivity of 281.7 mM(-1) s(-1), which was much higher than that of MnFe2O4 nanoparticles (67.5 mM 1 s(-1)). After being grafted with the targeted CD105 peptide CL 1555, the nanomicelles can combine TVECs specifically and make the labeled TVECs dark in T2-weighted MR imaging. With the passage on, the Mn(2+) ions were released from MnFe2O4 and the size decreased gradually, making the signal intensity of the second and third passage of labeled TVECs increased in T1-weighted MR imaging. Our results demonstrate that CL-poly(ethylene glycol)-MnFe2O4 can conjugate TVECs and induce dark and bright contrast in MR imaging, and act as a novel molecular probe for T1- and T2-enhanced MR imaging of tumor angiogenesis.
Keywords: CD105; CL 1555; CL-PEG-MnFe2O4; TVECs; tumor angio genesis.
Figures







References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Morgan-Parkes JH. Metastases: mechanisms, pathways, and cascades. AJR Am J Roentgenol. 1995;164(5):1075–1082. - PubMed
-
- Blood CH, Zetter BR. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990;1032(1):89–118. - PubMed
-
- Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–340. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

