Integrating Genomic Resources with Electronic Health Records using the HL7 Infobutton Standard
- PMID: 27579472
- PMCID: PMC5052552
- DOI: 10.4338/ACI-2016-04-RA-0058
Integrating Genomic Resources with Electronic Health Records using the HL7 Infobutton Standard
Abstract
Background: The Clinical Genome Resource (ClinGen) Electronic Health Record (EHR) Workgroup aims to integrate ClinGen resources with EHRs. A promising option to enable this integration is through the Health Level Seven (HL7) Infobutton Standard. EHR systems that are certified according to the US Meaningful Use program provide HL7-compliant infobutton capabilities, which can be leveraged to support clinical decision-making in genomics.
Objectives: To integrate genomic knowledge resources using the HL7 infobutton standard. Two tactics to achieve this objective were: (1) creating an HL7-compliant search interface for ClinGen, and (2) proposing guidance for genomic resources on achieving HL7 Infobutton standard accessibility and compliance.
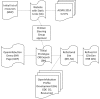
Methods: We built a search interface utilizing OpenInfobutton, an open source reference implementation of the HL7 Infobutton standard. ClinGen resources were assessed for readiness towards HL7 compliance. Finally, based upon our experiences we provide recommendations for publishers seeking to achieve HL7 compliance.
Results: Eight genomic resources and two sub-resources were integrated with the ClinGen search engine via OpenInfobutton and the HL7 infobutton standard. Resources we assessed have varying levels of readiness towards HL7-compliance. Furthermore, we found that adoption of standard terminologies used by EHR systems is the main gap to achieve compliance.
Conclusion: Genomic resources can be integrated with EHR systems via the HL7 Infobutton standard using OpenInfobutton. Full compliance of genomic resources with the Infobutton standard would further enhance interoperability with EHR systems.
Keywords: Clinical information systems; HL7; clinical decision support; genetics; standards adoption.
Conflict of interest statement
The authors declare that they have no conflicts of interest in the research.
Figures



References
-
- Zook J, Salit M. Chapter 23 – Genomic Reference Materials for Clinical Applications. Pfeifer SK, editor. Clinical Genomics [Internet]. Boston: Academic Press; 2015. p. 393–402. Available from: http://www.sciencedirect.com/science/article/pii/B978012404748800023X
-
- Biesecker LG, Green RC. Diagnostic Clinical Genome and Exome Sequencing. N Engl J Med 2014; 370(25):2418–2425. - PubMed
-
- ACMG Board of Directors. Clinical utility of genetic and genomic services: a position statement of the American College of Medical Genetics and Genomics. Genet Med 2015; 17(6):505–507. - PubMed
-
- Leape LL, Bates DW, Cullen DJ, Cooper J, Demonaco HJ, Gallivan T, Hallisey R, Ives J, Laird N, Laffel G. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA 1995; 274(1):35–43. - PubMed
-
- Institute of Medicine (U.S.), Committee on Quality of Health Care in America. Crossing the quality chasm a new health system for the 21st century [Internet]. Washington, D.C.: National Academy Press; 2001. Available from: http://site.ebrary.com/id/10032412
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

