"Act on Threes" Paradigm for Treatment Intensification of Type 2 Diabetes in Managed Care: Results of a Randomized Controlled Study with an Educational Intervention Targeting Improved Glycemic Control
- PMID: 27579824
- PMCID: PMC10397621
- DOI: 10.18553/jmcp.2016.22.9.1028
"Act on Threes" Paradigm for Treatment Intensification of Type 2 Diabetes in Managed Care: Results of a Randomized Controlled Study with an Educational Intervention Targeting Improved Glycemic Control
Abstract
Background: Clinical inertia, which has been defined as the recognition of a problem with a patient's management but failing to act, is a concern in type 2 diabetes (T2D) because it places the patient at risk of diabetes-related complications. Despite managed care organizations making significant investment in this area, little is known about the impact of educational programs aimed at aligning patients and their physicians with diabetes guidelines and thus overcoming clinical inertia.
Objective: To assess the impact of an educational intervention specifically designed to align patients and their physicians with 2012 American Diabetes Association (ADA) guidelines on glycated hemoglobin (A1c) testing frequency and insulin initiation.
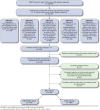
Methods: The "Act on Threes" educational intervention was a 12-month, randomized controlled prospective study that included Medicare Advantage patients aged 18-85 years with T2D, who received ≥ 3 oral antidiabetes drugs (OADs) and/or had A1c not at goal and/or had no recent A1c evaluation over 12 months, as identified through the analysis of administrative claims data (May 1, 2011-April 30, 2013) from the Humana database. Identified patients were randomized 3:1 to receive the Act on Threes educational intervention in conjunction with standard care (intervention group) or standard care alone (control group). For the educational intervention, patients and physicians were simultaneously mailed general and targeted information aimed at aligning them to 3 vital aspects of A1c control: timely measurement of A1c every 3 months; timely treatment intensification to meet A1c goals with treatment intensification every 3 months if A1c is not at goal; and insulin initiation when appropriate, including patients receiving ≥ 3 OADs with A1c not at goal. Control patients were only enrolled if the treating physician was not involved in the care of any patients in the intervention group. The primary outcome measures were A1c testing frequency based on the ADA standard for compliance of ≥ 2 tests per year and insulin initiation in the 12-month postintervention period. A1c levels were evaluated for the subgroup of patients with available A1c measurements in the pre- and postintervention periods. Descriptive statistics were used to analyze differences between the intervention and control groups. Multiple logistic regression analysis was used to identify determinants of insulin initiation in the full study cohort.
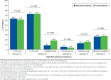
Results: 6,243 patients (mean age 70 years; 43.5% female) were identified: 4,555 were randomized to the intervention group and 1,688 to the control group. The percentage of patients with ≥ 2 A1c tests per year was not significantly different postintervention for patients in the intervention and control groups (47.7% vs. 46.8%, respectively; P = 0.995). Intriguingly, the frequency of A1c testing increased significantly from pre- to postintervention in the intervention and control groups. Change in A1c level from pre- to postintervention was also similar for the 2 groups (P = 0.240). A similar percentage of patients in the intervention and control groups initiated insulin during the postintervention period (6.3% vs. 7.6%, respectively; P = 0.059).
Conclusions: This randomized controlled study demonstrated that, compared with standard care, the Act on Threes educational intervention combined with standard care did not result in any significant differences in the frequency of A1c testing or in the initiation of insulin in patients with T2D. These findings are in contrast to uncontrolled comparative studies showing significant improvements in outcomes postintervention and reinforce the importance of study design in evaluating the effectiveness of educational programs.
Disclosures: This study was funded by Sanofi U.S. Reynolds, Davis, Kamble, and Uribe are employees of Comprehensive Health Insights, which was contracted by Sanofi U.S. to conduct, publish, and present this study. Bieszk and Wei are employees of Sanofi U.S. Reynolds and Uribe provided expertise and key clinical insights for the study design and methodology, provided interpretations of the data, contributed to the discussion, and reviewed the manuscript. Bieszk and Wei codeveloped the study design, researched data, contributed to discussion, and reviewed the manuscript. Davis and Kamble collected the data, provided study design, clinical insights, statistical and analytic reflections of the data, drafted the study reports, and reviewed the manuscript. All authors had full access to all the data in the study. Reynolds is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments: Writing/editorial support in the preparation of this manuscript, which was funded by Sanofi U.S., was provided by Rosalie Gadiot, PhD, of Excerpta Medica, who wrote the initial draft of the manuscript.
Conflict of interest statement
This study was funded by Sanofi U.S. Reynolds, Davis, Kamble, and Uribe are employees of Comprehensive Health Insights, which was contracted by Sanofi U.S. to conduct, publish, and present this study. Bieszk and Wei are employees of Sanofi U.S.
Reynolds and Uribe provided expertise and key clinical insights for the study design and methodology, provided interpretations of the data, contributed to the discussion, and reviewed the manuscript. Bieszk and Wei codeveloped the study design, researched data, contributed to discussion, and reviewed the manuscript. Davis and Kamble collected the data, provided study design, clinical insights, statistical and analytic reflections of the data, drafted the study reports, and reviewed the manuscript. All authors had full access to all the data in the study. Reynolds is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- Stolar MW, Hoogwerf BJ, Gorshow SM, Boyle PJ, Wales DO. Managing type 2 diabetes: going beyond glycemic control. J Manag Care Pharm. 2008;14(5 Suppl B):S2-19. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2008.14.S5-B.1. - DOI - PubMed
-
- Shogbon AO, Levy SB. Intensive glucose control in the management of diabetes mellitus and inpatient hyperglycemia. Am J Health Syst Pharm. 2010;67(10):798-805. - PubMed
-
- Hanefeld M. Use of insulin in type 2 diabetes: what we learned from recent clinical trials on the benefits of early insulin initiation. Diabetes Metab. 2014;40:391-99. Available at: http://www.diabet-metabolism.com/article/S1262-3636(14)00139-6/pdf. Accessed July 18, 2016. - PubMed
-
- Smolen HJ, Murphy DR, Gahn JC, Yu X, Curtis BH. The evaluation of clinical and cost outcomes associated with earlier initiation of insulin in patients with type 2 diabetes mellitus. J Manag Care Spec Pharm. 2014;20(9):968-84. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2014.20.9.968. - DOI - PMC - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care. 2012;35(6):1364-79. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3357214/pdf/1364.pdf. Accessed July 18, 2016. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous