Regorafenib (Stivarga) pharmacologically targets epithelial-mesenchymal transition in colorectal cancer
- PMID: 27580057
- PMCID: PMC5325431
- DOI: 10.18632/oncotarget.11636
Regorafenib (Stivarga) pharmacologically targets epithelial-mesenchymal transition in colorectal cancer
Abstract
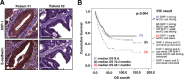
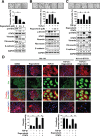
Epithelial-to-mesenchymal transition (EMT) is well-known to evoke cancer invasion/metastasis, leading to a high frequency of mortality in patients with metastatic colorectal cancer (mCRC). Protein tyrosine phosphatase (PTPase)-targeted therapy has been identified as a novel cancer therapeutic. Previously, we proved that sorafenib with anti-EMT potency prevents TGF-β1-induced EMT/invasion by directly activating SH2-domain-containing phosphatase 1 (SHP-1)-dependent p-STAT3Tyr705 suppression in hepatocellular carcinoma. Regorafenib has a closely related chemical structure as sorafenib and is approved for the pharmacotherapy of mCRC. Herein, we evaluate whether regorafenib activates PTPase SHP-1 in the same way as sorafenib to abolish EMT-related invasion/metastasis in CRC. Notably, regorafenib exerted potent anti-EMT activity to curb TGF-β1-induced EMT/invasion in vitro as well inhibited lung metastatic outgrowth of SW480 mesenchymal cells in vivo. Mechanistically, regorafenib-enhanced SHP-1 activity significantly impeded TGF-β1-induced EMT/invasion via low p-STAT3Tyr705 level as proved by a SHP-1 inhibitor or siRNA-mediated SHP-1 depletion. Conversely, overexpression of SHP-1 further enhanced the inhibitory effects of regorafenib on TGF-β1-induced p-STAT3Tyr705 and EMT/invasion. Regorafenib directly activates SHP-1 by potently relieving the autoinhibited N-SH2 domain of SHP-1 to inhibit TGF-β1-induced p-STAT3Tyr705 and EMT/invasion. Importantly, the clinical evidence indicated that SHP-1 was positively correlated with E-cadherin and that significantly determined the overall survival of CRC patients. This result further confirms our in vitro data that SHP-1 is a negative regulatory PTPase in EMT regulation and serves as a pharmacological target for mCRC therapy. Collectively, activating PTPase SHP-1 by regorafenib focusing on its anti-EMT activity might be a useful pharmacotherapy for mCRC.
Keywords: CRC; EMT; SHP-1; STAT3; regorafenib.
Conflict of interest statement
We have no potential conflicts of interest to disclose.
Figures



Similar articles
-
SHP-1 is a target of regorafenib in colorectal cancer.Oncotarget. 2014 Aug 15;5(15):6243-51. doi: 10.18632/oncotarget.2191. Oncotarget. 2014. PMID: 25071018 Free PMC article.
-
Pharmacological Targeting SHP-1-STAT3 Signaling Is a Promising Therapeutic Approach for the Treatment of Colorectal Cancer.Neoplasia. 2015 Sep;17(9):687-696. doi: 10.1016/j.neo.2015.08.007. Neoplasia. 2015. PMID: 26476076 Free PMC article.
-
Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article.
-
Regorafenib: A Review in Metastatic Colorectal Cancer.Drugs. 2018 Jul;78(11):1133-1144. doi: 10.1007/s40265-018-0938-y. Drugs. 2018. PMID: 29943375 Review.
-
Regorafenib.Recent Results Cancer Res. 2018;211:45-56. doi: 10.1007/978-3-319-91442-8_3. Recent Results Cancer Res. 2018. PMID: 30069758 Review.
Cited by
-
A marginal anticancer effect of regorafenib on pancreatic carcinoma cells in vitro, ex vivo, and in vivo.Naunyn Schmiedebergs Arch Pharmacol. 2017 Nov;390(11):1125-1134. doi: 10.1007/s00210-017-1412-1. Epub 2017 Aug 4. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 28779210
-
A framework for the development of effective anti-metastatic agents.Nat Rev Clin Oncol. 2019 Mar;16(3):185-204. doi: 10.1038/s41571-018-0134-8. Nat Rev Clin Oncol. 2019. PMID: 30514977 Free PMC article.
-
Therapy for Cancer: Strategy of Combining Anti-Angiogenic and Target Therapies.Front Cell Dev Biol. 2017 Dec 7;5:101. doi: 10.3389/fcell.2017.00101. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 29270405 Free PMC article. Review.
-
Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers.Exp Mol Med. 2019 Sep 24;51(9):1-15. doi: 10.1038/s12276-019-0308-1. Exp Mol Med. 2019. PMID: 31551425 Free PMC article. Review.
-
Alteration of SHP-1/p-STAT3 Signaling: A Potential Target for Anticancer Therapy.Int J Mol Sci. 2017 Jun 8;18(6):1234. doi: 10.3390/ijms18061234. Int J Mol Sci. 2017. PMID: 28594363 Free PMC article. Review.
References
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. - PubMed
-
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. - PubMed
-
- Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, Lederle W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–1331. - PubMed
-
- Chen HN, Yuan K, Xie N, Wang K, Huang Z, Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG, Huang C. PDLIM1 stabilizes the E-cadherin/beta-catenin complex to prevent epithelial-mesenchymal transition and metastatic potential of colorectal cancer cells. Cancer Res. 2016;76:1122–1134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

