Cyclin-dependent kinase inhibitor flavopiridol promotes remyelination in a cuprizone induced demyelination model
- PMID: 27580304
- PMCID: PMC5053583
- DOI: 10.1080/15384101.2016.1220458
Cyclin-dependent kinase inhibitor flavopiridol promotes remyelination in a cuprizone induced demyelination model
Abstract
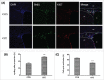
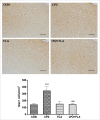
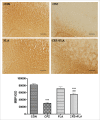
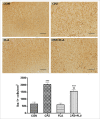
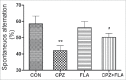
The cuprizone (CPZ) model has been widely used for the studies of de-and remyelination. The CPZ-exposed mice show oligodendrocyte precursor cells (OPCs) increase and mature oligodendrocytes decrease, suggesting an imbalance between proliferation and differentiation of OPCs. In the first experiment of this study, we examined the expression of cell cycle related genes in brains of mice following CPZ administration for 5 weeks by means of microarray assay. In addition, we performed a double labeling of BrdU and Ki-67 to calculate cell cycle exit index in the mice. Our results showed that CPZ administration up-regulated the expression of 16 cell cycle related genes, but down-regulated the expression of only one in the prefrontal cortex (PFC) of mice compared to control group. The treatment inhibited potential precursor cells exit from cell cycle. In the second experiment, we evaluated effects of a CDK inhibitor flavopiridol (FLA) on CPZ-induced neuropathological changes and spatial working memory impairment in mice.FLA treatment for one week effectively attenuated the CPZ-induced increases in NG2 positive cells, microglia and astrocytes, alleviated the concurrent mature oligodendrocyte loss and myelin breakdown, and improved spatial working memory deficit in the CPZ-exposed mice. These results suggest that CPZ-induced neuropathological changes involve in dysregulation of cell cycle related genes. The therapeutic effects of FLA on CPZ-exposed mice may be related to its ability of cell cycle inhibition.
Keywords: astrocyte; cell cycle; cognition; cuprizone; flavopiridol; microglia; oligodendrocyte.
Figures


Similar articles
-
Galectin-3 controls the response of microglial cells to limit cuprizone-induced demyelination.Neurobiol Dis. 2014 Feb;62:441-55. doi: 10.1016/j.nbd.2013.10.023. Epub 2013 Oct 31. Neurobiol Dis. 2014. PMID: 24184798
-
Thyroid hormone alleviates demyelination induced by cuprizone through its role in remyelination during the remission period.Exp Biol Med (Maywood). 2015 Sep;240(9):1183-96. doi: 10.1177/1535370214565975. Epub 2015 Jan 10. Exp Biol Med (Maywood). 2015. PMID: 25577802 Free PMC article.
-
Golli myelin basic proteins stimulate oligodendrocyte progenitor cell proliferation and differentiation in remyelinating adult mouse brain.Glia. 2012 Jul;60(7):1078-93. doi: 10.1002/glia.22336. Epub 2012 Mar 23. Glia. 2012. PMID: 22447683
-
The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis.Glia. 2022 Jul;70(7):1215-1250. doi: 10.1002/glia.24148. Epub 2022 Feb 2. Glia. 2022. PMID: 35107839 Free PMC article. Review.
-
Five Decades of Cuprizone, an Updated Model to Replicate Demyelinating Diseases.Curr Neuropharmacol. 2019;17(2):129-141. doi: 10.2174/1570159X15666170717120343. Curr Neuropharmacol. 2019. PMID: 28714395 Free PMC article. Review.
Cited by
-
Dicer deficiency affects microglial function during demyelination and impairs remyelination.Neurobiol Dis. 2025 May;208:106879. doi: 10.1016/j.nbd.2025.106879. Epub 2025 Mar 20. Neurobiol Dis. 2025. PMID: 40120829 Free PMC article.
-
Anti-Inflammatory Effects of Miyako Bidens pilosa in a Mouse Model of Amyotrophic Lateral Sclerosis and Lipopolysaccharide-Stimulated BV-2 Microglia.Int J Mol Sci. 2023 Sep 5;24(18):13698. doi: 10.3390/ijms241813698. Int J Mol Sci. 2023. PMID: 37762010 Free PMC article.
-
Low-field magnetic stimulation improved cuprizone-induced depression-like symptoms and demyelination in female mice.Exp Ther Med. 2022 Mar;23(3):210. doi: 10.3892/etm.2022.11133. Epub 2022 Jan 7. Exp Ther Med. 2022. PMID: 35126713 Free PMC article.
-
Remyelination in Multiple Sclerosis: Findings in the Cuprizone Model.Int J Mol Sci. 2022 Dec 17;23(24):16093. doi: 10.3390/ijms232416093. Int J Mol Sci. 2022. PMID: 36555733 Free PMC article. Review.
-
HDAC1 dysregulation induces aberrant cell cycle and DNA damage in progress of TDP-43 proteinopathies.EMBO Mol Med. 2020 Jun 8;12(6):e10622. doi: 10.15252/emmm.201910622. Epub 2020 May 25. EMBO Mol Med. 2020. PMID: 32449313 Free PMC article.
References
-
- Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treatment 2011; 2011:325789; PMID:22937264; http://dx.doi.org/10.1155/2011/325789 - DOI - PMC - PubMed
-
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE. Degeneration and impaired regeneration of gray matter oligo- dendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 2013; 16:571-9; PMID:23542689; http://dx.doi.org/10.1038/nn.3357 - DOI - PMC - PubMed
-
- Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P. Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 2014; 47:485-505; PMID:25445182; http://dx.doi.org/10.1016/j.neubiorev.2014.10.004 - DOI - PubMed
-
- Skripuletz T, Gudi V, Hackstette D, Stangel M. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol 2011; 26:1585-97 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
