Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer's and Parkinson's Diseases
- PMID: 27580631
- PMCID: PMC5088528
- DOI: 10.1101/cshperspect.a023630
Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer's and Parkinson's Diseases
Abstract
Experimental data indicate that transneuronal propagation of abnormal protein aggregates in neurodegenerative proteinopathies, such as sporadic Alzheimer's disease (AD) and Parkinson's disease (PD), is capable of a self-propagating process that leads to a progression of neurodegeneration and accumulation of prion-like particles. The mechanisms by which misfolded tau and α-synuclein possibly spread from one involved nerve cell to the next in the neuronal chain to induce abnormal aggregation are still unknown. Based on findings from studies of human autopsy cases, we review potential pathways and mechanisms related to axonal and transneuronal dissemination of tau (sporadic AD) and α-synuclein (sporadic PD) aggregates between anatomically interconnected regions.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
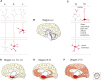
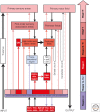

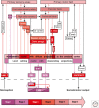
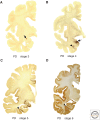
References
-
- Agnati LF, Bjekle B, Fuxe K. 1995. Volume versus wiring transmission in the brain: A new theoretical frame of neuropsychopharmacology. Med Res Rev 15: 33–45. - PubMed
-
- Aguzzi A. 2009. Beyond the prion principle. Nature 459: 924–925. - PubMed
-
- Aguzzi A, Calella AM. 2009. Prions: Protein aggregation and infectious diseases. Physiol Rev 89: 1105–1152. - PubMed
-
- Aguzzi A, Rajendran L. 2009. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64: 783–790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
