Bach2-Batf interactions control Th2-type immune response by regulating the IL-4 amplification loop
- PMID: 27581382
- PMCID: PMC5025763
- DOI: 10.1038/ncomms12596
Bach2-Batf interactions control Th2-type immune response by regulating the IL-4 amplification loop
Abstract
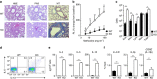
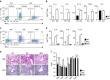
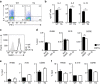
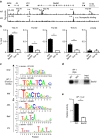
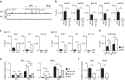
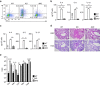
Although Bach2 has an important role in regulating the Th2-type immune response, the underlying molecular mechanisms remain unclear. We herein demonstrate that Bach2 associates with Batf and binds to the regulatory regions of the Th2 cytokine gene loci. The Bach2-Batf complex antagonizes the recruitment of the Batf-Irf4 complex to AP-1 motifs and suppresses Th2 cytokine production. Furthermore, we find that Bach2 regulates the Batf and Batf3 expressions via two distinct pathways. First, Bach2 suppresses the maintenance of the Batf and Batf3 expression through the inhibition of IL-4 production. Second, the Bach2-Batf complex directly binds to the Batf and Batf3 gene loci and reduces transcription by interfering with the Batf-Irf4 complex. These findings suggest that IL-4 and Batf form a positive feedback amplification loop to induce Th2 cell differentiation and the subsequent Th2-type immune response, and Bach2-Batf interactions are required to prevent an excessive Th2 response.
Figures


References
-
- Reiner S. L. Development in motion: helper T cells at work. Cell 129, 33–36 (2007). - PubMed
-
- Sakaguchi S., Yamaguchi T., Nomura T. & Ono M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008). - PubMed
-
- Korn T., Bettelli E., Oukka M. & Kuchroo V. K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

