Hypoxia upregulates neutrophil degranulation and potential for tissue injury
- PMID: 27581620
- PMCID: PMC5099189
- DOI: 10.1136/thoraxjnl-2015-207604
Hypoxia upregulates neutrophil degranulation and potential for tissue injury
Abstract
Background: The inflamed bronchial mucosal surface is a profoundly hypoxic environment. Neutrophilic airway inflammation and neutrophil-derived proteases have been linked to disease progression in conditions such as COPD and cystic fibrosis, but the effects of hypoxia on potentially harmful neutrophil functional responses such as degranulation are unknown.
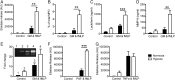
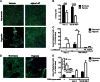
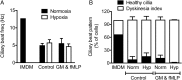
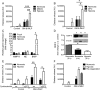
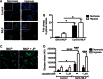
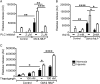
Methods and results: Following exposure to hypoxia (0.8% oxygen, 3 kPa for 4 h), neutrophils stimulated with inflammatory agonists (granulocyte-macrophage colony stimulating factor or platelet-activating factor and formylated peptide) displayed a markedly augmented (twofold to sixfold) release of azurophilic (neutrophil elastase, myeloperoxidase), specific (lactoferrin) and gelatinase (matrix metalloproteinase-9) granule contents. Neutrophil supernatants derived under hypoxic but not normoxic conditions induced extensive airway epithelial cell detachment and death, which was prevented by coincubation with the antiprotease α-1 antitrypsin; both normoxic and hypoxic supernatants impaired ciliary function. Surprisingly, the hypoxic upregulation of neutrophil degranulation was not dependent on hypoxia-inducible factor (HIF), nor was it fully reversed by inhibition of phospholipase C signalling. Hypoxia augmented the resting and cytokine-stimulated phosphorylation of AKT, and inhibition of phosphoinositide 3-kinase (PI3K)γ (but not other PI3K isoforms) prevented the hypoxic upregulation of neutrophil elastase release.
Conclusion: Hypoxia augments neutrophil degranulation and confers enhanced potential for damage to respiratory airway epithelial cells in a HIF-independent but PI3Kγ-dependent fashion.
Keywords: Airway Epithelium; COPD ÀÜ Mechanisms; Cystic Fibrosis; Innate Immunity; Neutrophil Biology; Respiratory Infection.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Comment in
-
Neutrophils and tissue damage: is hypoxia the key to excessive degranulation?Thorax. 2016 Nov;71(11):977-978. doi: 10.1136/thoraxjnl-2016-208879. Epub 2016 Sep 1. Thorax. 2016. PMID: 27586870 No abstract available.
References
-
- Abraham E, Carmody A, Shenkar R, et al. . Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol 2000;279:L1137–45. - PubMed
-
- Worthen GS, Haslett C, Rees AJ, et al. . Neutrophil-mediated pulmonary vascular injury. Synergistic effect of trace amounts of lipopolysaccharide and neutrophil stimuli on vascular permeability and neutrophil sequestration in the lung. Am Rev Respir Dis 1987;136:19–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
