Use of safety-engineered devices by healthcare workers for intravenous and/or phlebotomy procedures in healthcare settings: a systematic review and meta-analysis
- PMID: 27581947
- PMCID: PMC5007867
- DOI: 10.1186/s12913-016-1705-y
Use of safety-engineered devices by healthcare workers for intravenous and/or phlebotomy procedures in healthcare settings: a systematic review and meta-analysis
Abstract
Background: The acquisition of needle-stick injuries (NSI) in a healthcare setting poses an occupational hazard of transmitting blood-borne pathogens from patients to healthcare workers (HCWs). The objective of this study was to systematically review the evidence about the efficacy and safety of using safety-engineered intravenous devices and safety-engineered phlebotomy devices by HCWs.
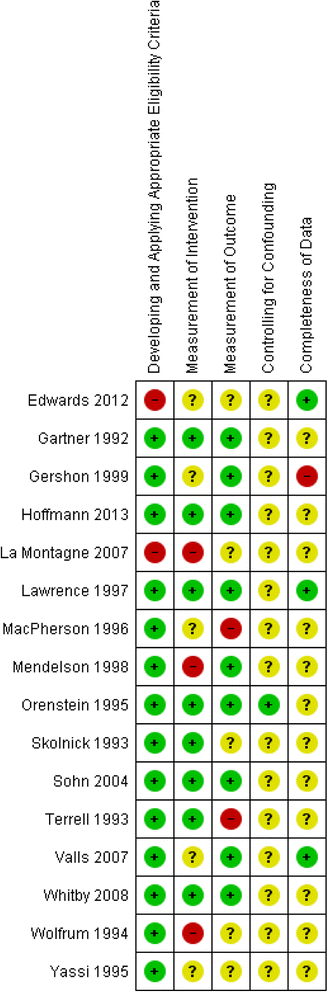
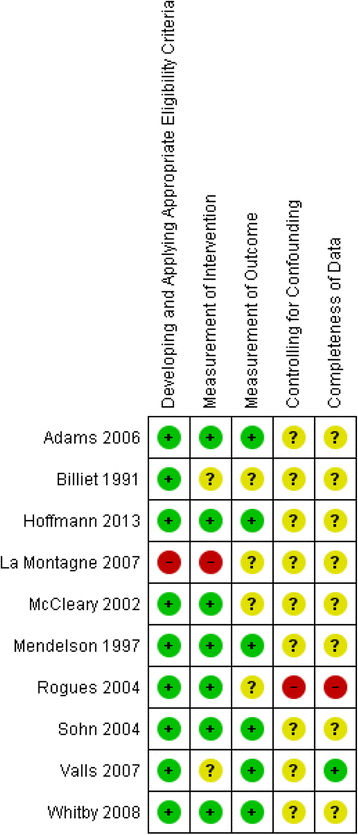
Methods: We included randomized and non-randomized studies comparing safety-engineered devices to conventional/standard devices that lack safety features for delivering intravenous injections and/or for blood-withdrawal procedures (phlebotomy). The outcomes of interest included NSI rates, and blood-borne infections rates among HCWs and patients. We conducted an extensive literature search strategy using the OVID interface in October 2013. We followed the standard methods for study selection and data abstraction. When possible, we conducted meta-analyses using a random-effects model. We used the GRADE methodology to assess the quality of evidence by outcome.
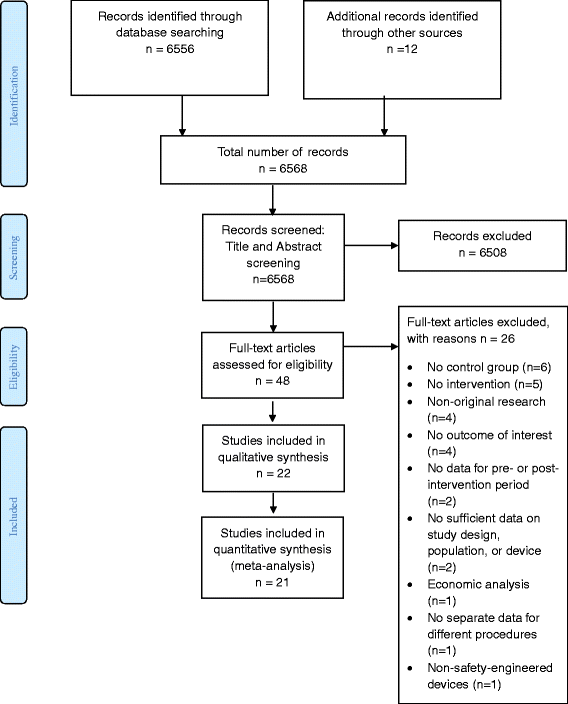
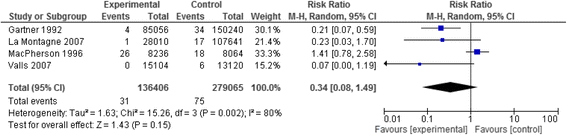
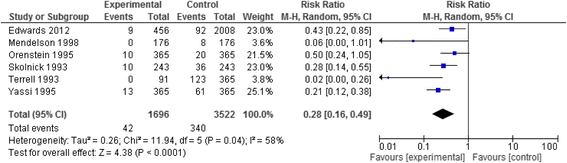
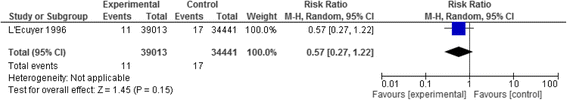
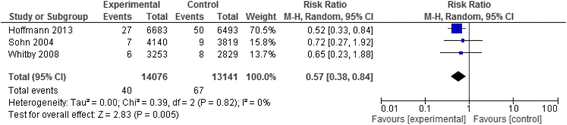
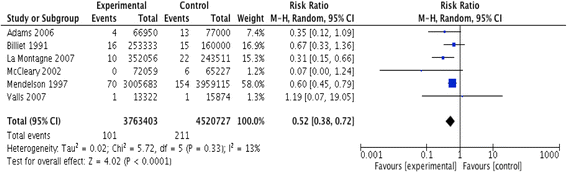
Results: We identified twenty-two eligible studies: Twelve assessed safety-engineered devices for intravenous procedures, five for phlebotomy procedures, and five for both. Twenty-one of those studies were observational while one was a randomized trial. All studies assessed the reduction in NSIs among HCWs. For safety-engineered intravenous devices, the pooled relative risk for NSI per HCW was 0.28 [0.13, 0.59] (moderate quality evidence). The pooled relative risk for NSI per device used or procedure performed was 0.34 [0.08,1.49] (low quality evidence). For safety-engineered phlebotomy devices, the pooled relative risk for NSI per HCW was 0.57 [0.38, 0.84] (moderate quality evidence). The pooled relative risk for NSI per device used or procedure performed was 0.53 [0.43,0.65] (moderate quality evidence). We identified no studies assessing the outcome of blood-borne infections among healthcare workers or patients.
Conclusion: There is moderate-quality evidence that the use of safety-engineered devices in intravenous injections and infusions, and phlebotomy (blood-drawing) procedures reduces NSI rates of HCWs.
Keywords: Blood-borne pathogens; Healthcare setting; Healthcare workers; Intravenous; Meta-analysis; Needle-stick injuries; Phlebotomy; Safety-engineered devices; Systematic review.
Figures


References
-
- Jeffress CN. Occupational Exposure to Bloodborne Pathogens; Needlestick and Other Sharps Injuries; Final Rule. In: Administration OSH, ed. United States Department of Labor Website; 2001. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=FEDERAL_R.... - PubMed
-
- Vaughan ARaP. The World Health Report 2002. In: Campanini B, ed. Reducing Risks, Promoting Healthy Life: World Health Organization; 2002.
-
- Centers for Disease Control and Prevention (CDC) Evaluation of safety devices for preventing percutaneous injuries among health-care workers during phlebotomy procedures -- Minneapolis-St. Paul, New York City, and San Francisco, 1993-1995. MMWR Morb Mortal Wkly Rep. 1997;46(2):21–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

