Packaging and Prefusion Stabilization Separately and Additively Increase the Quantity and Quality of Respiratory Syncytial Virus (RSV)-Neutralizing Antibodies Induced by an RSV Fusion Protein Expressed by a Parainfluenza Virus Vector
- PMID: 27581977
- PMCID: PMC5068507
- DOI: 10.1128/JVI.01196-16
Packaging and Prefusion Stabilization Separately and Additively Increase the Quantity and Quality of Respiratory Syncytial Virus (RSV)-Neutralizing Antibodies Induced by an RSV Fusion Protein Expressed by a Parainfluenza Virus Vector
Abstract
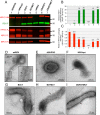
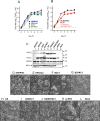
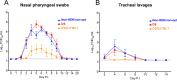
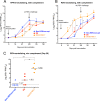
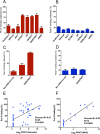
Human respiratory syncytial virus (RSV) and human parainfluenza virus type 3 (HPIV3) are major pediatric respiratory pathogens that lack vaccines. A chimeric bovine/human PIV3 (rB/HPIV3) virus expressing the unmodified, wild-type (wt) RSV fusion (F) protein from an added gene was previously evaluated in seronegative children as a bivalent intranasal RSV/HPIV3 vaccine, and it was well tolerated but insufficiently immunogenic for RSV F. We recently showed that rB/HPIV3 expressing a partially stabilized prefusion form (pre-F) of RSV F efficiently induced "high-quality" RSV-neutralizing antibodies, defined as antibodies that neutralize RSV in vitro without added complement (B. Liang et al., J Virol 89:9499-9510, 2015, doi:10.1128/JVI.01373-15). In the present study, we modified RSV F by replacing its cytoplasmic tail (CT) domain or its CT and transmembrane (TM) domains (TMCT) with counterparts from BPIV3 F, with or without pre-F stabilization. This resulted in RSV F being packaged in the rB/HPIV3 particle with an efficiency similar to that of RSV particles. Enhanced packaging was substantially attenuating in hamsters (10- to 100-fold) and rhesus monkeys (100- to 1,000-fold). Nonetheless, TMCT-directed packaging substantially increased the titers of high-quality RSV-neutralizing serum antibodies in hamsters. In rhesus monkeys, a strongly additive immunogenic effect of packaging and pre-F stabilization was observed, as demonstrated by 8- and 30-fold increases of RSV-neutralizing serum antibody titers in the presence and absence of added complement, respectively, compared to pre-F stabilization alone. Analysis of vaccine-induced F-specific antibodies by binding assays indicated that packaging conferred substantial stabilization of RSV F in the pre-F conformation. This provides an improved version of this well-tolerated RSV/HPIV3 vaccine candidate, with potently improved immunogenicity, which can be returned to clinical trials.
Importance: Human respiratory syncytial virus (RSV) and human parainfluenza virus type 3 (HPIV3) are major viral agents of acute pediatric bronchiolitis and pneumonia worldwide that lack vaccines. A bivalent intranasal RSV/HPIV3 vaccine candidate consisting of a chimeric bovine/human PIV3 (rB/HPIV3) strain expressing the RSV fusion (F) protein was previously shown to be well tolerated by seronegative children but was insufficiently immunogenic for RSV F. In the present study, the RSV F protein was engineered to be packaged efficiently into vaccine virus particles. This resulted in a significantly enhanced quantity and quality of RSV-neutralizing antibodies in hamsters and nonhuman primates. In nonhuman primates, this effect was strongly additive to the previously described stabilization of the prefusion conformation of the F protein. The improved immunogenicity of RSV F by packaging appeared to involve prefusion stabilization. These findings provide a potently more immunogenic version of this well-tolerated vaccine candidate and should be applicable to other vectored vaccines.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi:10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Murphy BR, Prince GA, Collins PL, Van Wyke Coelingh K, Olmsted RA, Spriggs MK, Parrott RH, Kim HW, Brandt CD, Chanock RM. 1988. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res 11:1–15. - PubMed
-
- Klein MI, Coviello S, Bauer G, Benitez A, Serra ME, Schiatti MP, Delgado MF, Melendi GA, Novalli L, Pena HG, Karron RA, Kleeberger SR, Polack FP. 2006. The impact of infection with human metapneumovirus and other respiratory viruses in young infants and children at high risk for severe pulmonary disease. J Infect Dis 193:1544–1551. doi:10.1086/503806. - DOI - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

