Virologic Differences Do Not Fully Explain the Diversification of Swine Influenza Viruses in the United States
- PMID: 27581984
- PMCID: PMC5105646
- DOI: 10.1128/JVI.01218-16
Virologic Differences Do Not Fully Explain the Diversification of Swine Influenza Viruses in the United States
Abstract
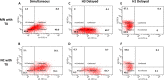
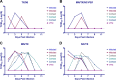
Influenza A(H1N1) viruses entered the U.S. swine population following the 1918 pandemic and remained genetically stable for roughly 80 years. In 1998, there was an outbreak of influenza-like illness among swine that was caused by A(H3N2) viruses containing the triple reassortant internal gene (TRIG) cassette. Following the TRIG cassette emergence, numerous reassortant viruses were isolated in nature, suggesting that the TRIG virus had an enhanced ability to reassort compared to the classical swine virus. The present study was designed to quantify the relative reassortment capacities of classical and TRIG swine viruses. Reverse genetic viruses were generated from the classical H1N1 virus A/swine/MN/37866/1999 (MN/99), the TRIG virus A/swine/NC/18161/2002 (NC/02), and a seasonal human H3N2 virus, A/TX/6/1996 (TX/96), to measure in vitro reassortment and growth potentials. After coinfection with NC/02 or MN/99 plus TX/96, H1/H3 double-positive cells were identified. Delayed TX/96 infection was fully excluded by both swine viruses. We then analyzed reassortant H3 viruses. Seventy-seven of 81 (95.1%) TX/96-NC/02 reassortants contained at least one polymerase gene segment from NC/02, whereas only 34 of 61 (55.7%) MN/99-TX/96 reassortants contained at least one polymerase gene segment from MN/99. Additionally, 38 of 81 (46.9%) NC/02-TX/96 reassortants contained all NC/02 polymerase gene segments, while none of the MN/99-TX/96 reassortants contained all MN/99 polymerase genes. There were 21 H3 reassortants between MN/99 and TX/96, compared to only 17 H3 reassortants between NC/02 and TX/96. Overall, the results indicate that there are no distinct differences in the ability of the TRIG to reassort with a human virus compared to the classical swine virus.
Importance: There appear to be no differences in the abilities of classical swine and TRIG swine viruses to exclude a second virus, suggesting that under the right circumstances both viruses have similar opportunities to reassort. The increased percentage of TRIG polymerase gene segments in reassortant H3 viruses indicates that these viruses may be more compatible with gene segments from other viruses; however, this needs to be investigated further. Nevertheless, the classical swine virus also showed the ability to reassort, suggesting that factors other than reassortment capacity alone are responsible for the different epidemiologies of TRIG and classical swine viruses. The post-TRIG diversity was likely driven by increased intensive farming practices rather than virologic properties. Our results indicate that host ecology can be a significant factor in viral evolution.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Webster RG. 2002. The importance of animal influenza for human disease. Vaccine 20(Suppl 2):S16–S20. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

