Production of Cytomegalovirus Dense Bodies by Scalable Bioprocess Methods Maintains Immunogenicity and Improves Neutralizing Antibody Titers
- PMID: 27581989
- PMCID: PMC5105651
- DOI: 10.1128/JVI.00463-16
Production of Cytomegalovirus Dense Bodies by Scalable Bioprocess Methods Maintains Immunogenicity and Improves Neutralizing Antibody Titers
Abstract
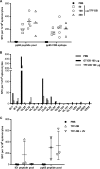
With the goal of developing a virus-like particle-based vaccine based on dense bodies (DB) produced by human cytomegalovirus (HCMV) infections, we evaluated scalable culture, isolation, and inactivation methods and applied technically advanced assays to determine the relative purity, composition, and immunogenicity of DB particles. Our results increase our understanding of the benefits and disadvantages of methods to recover immunogenic DB and inactivate contaminating viral particles. Our results indicate that (i) HCMV strain Towne replicates in MRC-5 fibroblasts grown on microcarriers, (ii) DB particles recovered from 2-bromo-5,6-dichloro-1-beta-d-ribofuranosyl benzimidazole riboside (BDCRB)-treated cultures and purified by tangential flow filtration (TFF-DB) or glycerol tartrate gradient sedimentation (GT-DB) constitute 92% or 98%, respectively, of all particles in the final product, (iii) epithelial cell-tropic DB particles are recovered from a single round of coinfection by AD169 and Towne strain viruses, consistent with complementation between the UL130 and UL131A expressed by these strains and restoration of gH/gL/UL128-UL131A (gH pentamer), (iv) equivalent neutralizing antibody titers are induced in mice following immunization with epithelial cell-tropic DB or gH pentamer-deficient DB preparations, (v) UV-inactivated residual virus in GT-DB or TFF-DB preparations retained immunogenicity and induced neutralizing antibody, preventing viral entry into epithelial cells, and (vi) GT-DB and TFF-DB induced cellular immune responses to multiple HCMV peptides. Collectively, this work provides a foundation for future development of DB as an HCMV-based particle vaccine.
Importance: Development of a vaccine to prevent congenital HCMV infection remains a high priority. Vaccination with human cytomegalovirus-derived noninfectious particles, or dense bodies, may constitute a safe vaccination strategy that mimics natural infection. The standard approach for purification of virus particles has been to use a multiple-step, complex gradient that presents a potential barrier to production scale-up and commercialization. In the study described here, we employed an approach that combines treatment with an antiviral terminase inhibitor and purification by a simplified process to produce a vaccine candidate providing broad antiviral humoral and cellular immunity as a foundation for future development.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures






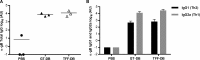
References
-
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG II, Rodland K, Wiley S, Britt W, Shenk T, Smith RD, Nelson JA. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J Virol 78:10960–10966. doi:10.1128/JVI.78.20.10960-10966.2004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

