Impairment of Wnt11 function leads to kidney tubular abnormalities and secondary glomerular cystogenesis
- PMID: 27582005
- PMCID: PMC5007805
- DOI: 10.1186/s12861-016-0131-z
Impairment of Wnt11 function leads to kidney tubular abnormalities and secondary glomerular cystogenesis
Abstract
Background: Wnt11 is a member of the Wnt family of secreted signals controlling the early steps in ureteric bud (UB) branching. Due to the reported lethality of Wnt11 knockout embryos in utero, its role in later mammalian kidney organogenesis remains open. The presence of Wnt11 in the emerging tubular system suggests that it may have certain roles later in the development of the epithelial ductal system.
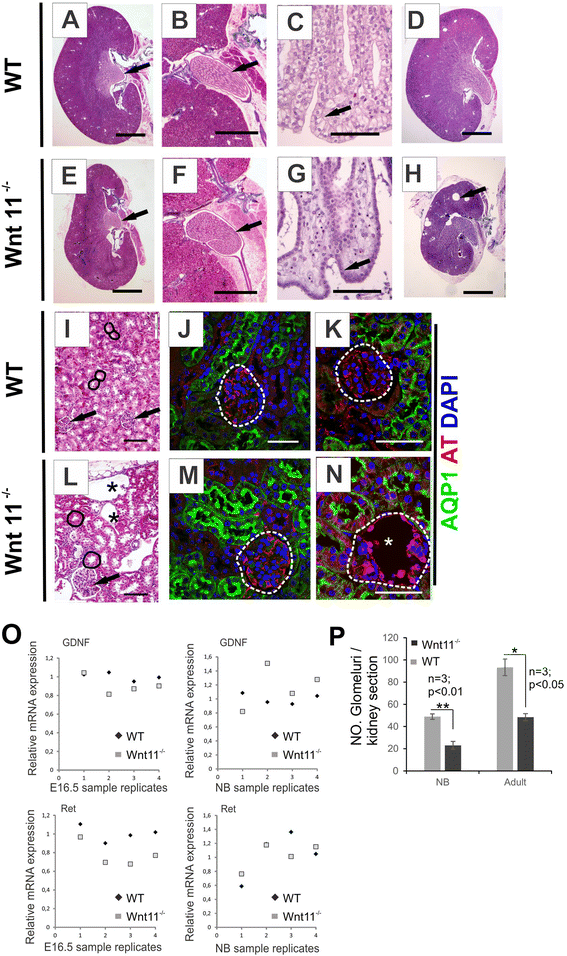
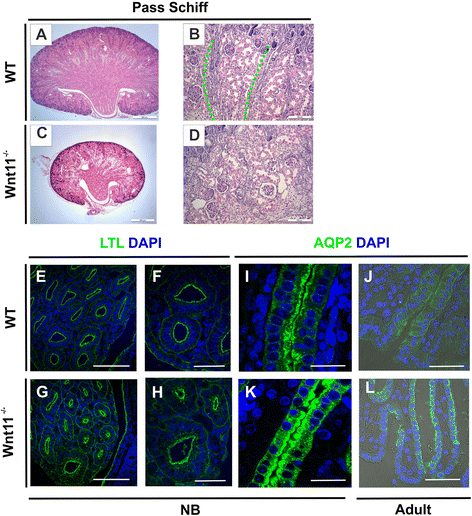
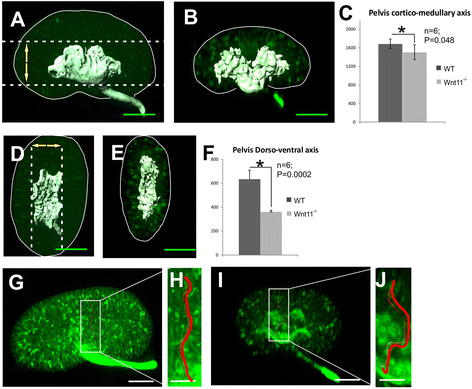
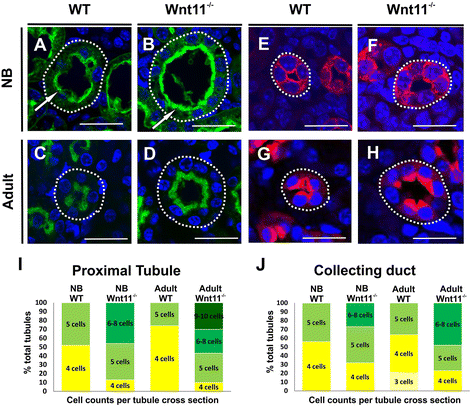
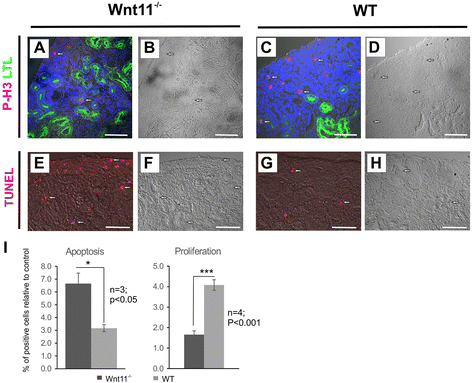
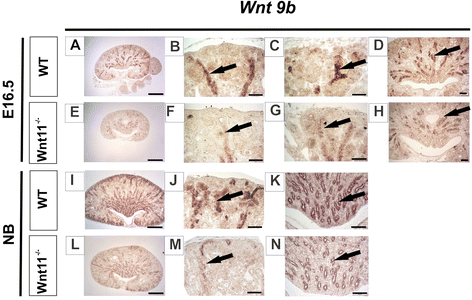
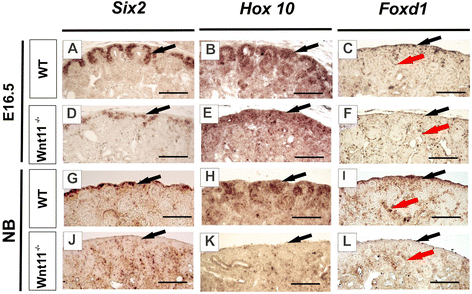
Results: The Wnt11 knockout allele was backcrossed with the C57Bl6 strain for several generations to address possible differences in penetrance of the kidney phenotypes. Strikingly, around one third of the null mice with this inbred background survived to the postnatal stages. Many of them also reached adulthood, but urine and plasma analyses pointed out to compromised kidney function. Consistent with these data the tubules of the C57Bl6 Wnt11 (-/-) mice appeared to be enlarged, and the optical projection tomography indicated changes in tubular convolution. Moreover, the C57Bl6 Wnt11 (-/-) mice developed secondary glomerular cysts not observed in the controls. The failure of Wnt11 signaling reduced the expression of several genes implicated in kidney development, such as Wnt9b, Six2, Foxd1 and Hox10. Also Dvl2, an important PCP pathway component, was downregulated by more than 90 % due to Wnt11 deficiency in both the E16.5 and NB kidneys. Since all these genes take part in the control of UB, nephron and stromal progenitor cell differentiation, their disrupted expression may contribute to the observed anomalies in the kidney tubular system caused by Wnt11 deficiency.
Conclusions: The Wnt11 signal has roles at the later stages of kidney development, namely in coordinating the development of the tubular system. The C57Bl6 Wnt11 (-/-) mouse generated here provides a model for studying the mechanisms behind tubular anomalies and glomerular cyst formation.
Keywords: Epithelial mesenchyme tissue interactions; Glomerular cysts; Tubule morphogenesis; Wnt signaling.
Figures

Similar articles
-
A secreted BMP antagonist, Cer1, fine tunes the spatial organization of the ureteric bud tree during mouse kidney development.PLoS One. 2011;6(11):e27676. doi: 10.1371/journal.pone.0027676. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22114682 Free PMC article.
-
Transcription Factor 21 Is Required for Branching Morphogenesis and Regulates the Gdnf-Axis in Kidney Development.J Am Soc Nephrol. 2018 Dec;29(12):2795-2808. doi: 10.1681/ASN.2017121278. Epub 2018 Oct 30. J Am Soc Nephrol. 2018. PMID: 30377232 Free PMC article.
-
Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis.Development. 2007 Jul;134(13):2397-405. doi: 10.1242/dev.02861. Epub 2007 May 23. Development. 2007. PMID: 17522159
-
Coordination of kidney organogenesis by Wnt signaling.Pediatr Nephrol. 2014 Apr;29(4):737-44. doi: 10.1007/s00467-013-2733-z. Epub 2014 Jan 21. Pediatr Nephrol. 2014. PMID: 24445433 Free PMC article. Review.
-
Kidney development: two tales of tubulogenesis.Curr Top Dev Biol. 2010;90:193-229. doi: 10.1016/S0070-2153(10)90005-7. Curr Top Dev Biol. 2010. PMID: 20691850 Review.
Cited by
-
Disruption of Core Planar Cell Polarity Signaling Regulates Renal Tubule Morphogenesis but Is Not Cystogenic.Curr Biol. 2017 Oct 23;27(20):3120-3131.e4. doi: 10.1016/j.cub.2017.09.011. Epub 2017 Oct 12. Curr Biol. 2017. PMID: 29033332 Free PMC article.
-
The multisystemic functions of FOXD1 in development and disease.J Mol Med (Berl). 2018 Aug;96(8):725-739. doi: 10.1007/s00109-018-1665-2. Epub 2018 Jun 29. J Mol Med (Berl). 2018. PMID: 29959475 Review.
-
Prolonged prenatal hypoxia selectively disrupts collecting duct patterning and postnatal function in male mouse offspring.J Physiol. 2018 Dec;596(23):5873-5889. doi: 10.1113/JP275918. Epub 2018 Jul 5. J Physiol. 2018. PMID: 29676801 Free PMC article.
-
The Wnt/PCP formin Daam1 drives cell-cell adhesion during nephron development.Cell Rep. 2021 Jul 6;36(1):109340. doi: 10.1016/j.celrep.2021.109340. Cell Rep. 2021. PMID: 34233186 Free PMC article.
-
Postnatal prolongation of mammalian nephrogenesis by excess fetal GDNF.Development. 2021 May 15;148(10):dev197475. doi: 10.1242/dev.197475. Epub 2021 May 25. Development. 2021. PMID: 34032268 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

