Nrf2-AKT interactions regulate heme oxygenase 1 expression in kidney epithelia during hypoxia and hypoxia-reoxygenation
- PMID: 27582105
- PMCID: PMC5130454
- DOI: 10.1152/ajprenal.00362.2016
Nrf2-AKT interactions regulate heme oxygenase 1 expression in kidney epithelia during hypoxia and hypoxia-reoxygenation
Abstract
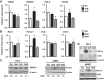
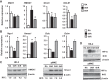
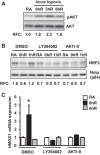
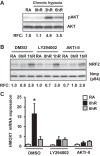
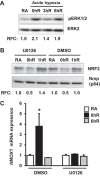
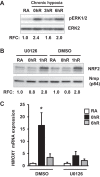
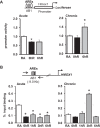
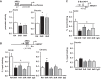
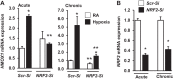
Ischemia-reperfusion (IR)-induced kidney injury is a major clinical problem, but its underlying mechanisms remain unclear. The transcription factor known as nuclear factor, erythroid 2-like 2 (NFE2L2 or Nrf2) is crucial for protection against oxidative stress generated by pro-oxidant insults. We have previously shown that Nrf2 deficiency enhances susceptibility to IR-induced kidney injury in mice and that its upregulation is protective. Here, we examined Nrf2 target antioxidant gene expression and the mechanisms of its activation in both human and murine kidney epithelia following acute (2 h) and chronic (12 h) hypoxia and reoxygenation conditions. We found that acute hypoxia modestly stimulates and chronic hypoxia strongly stimulates Nrf2 putative target HMOX1 expression, but not that of other antioxidant genes. Inhibition of AKT1/2 or ERK1/2 signaling blocked this induction; AKT1/2 but not ERK1/2 inhibition affected Nrf2 levels in basal and acute hypoxia-reoxygenation states. Unexpectedly, chromatin immunoprecipitation assays revealed reduced levels of Nrf2 binding at the distal AB1 and SX2 enhancers and proximal promoter of HMOX1 in acute hypoxia, accompanied by diminished levels of nuclear Nrf2. In contrast, Nrf2 binding at the AB1 and SX2 enhancers significantly but differentially increased during chronic hypoxia and reoxygenation, with reaccumulation of nuclear Nrf2 levels. Small interfering-RNA-mediated Nrf2 depletion attenuated acute and chronic hypoxia-inducible HMOX1 expression, and primary Nrf2-null kidney epithelia showed reduced levels of HMOX1 induction in response to both acute and chronic hypoxia. Collectively, our data demonstrate that Nrf2 upregulates HMOX1 expression in kidney epithelia through a distinct mechanism during acute and chronic hypoxia reoxygenation, and that both AKT1/2 and ERK1/2 signaling are required for this process.
Keywords: AKT1/2; ERK1/2; NRF2; acute kidney injury; antioxidant genes; ischemia-reperfusion.
Copyright © 2016 the American Physiological Society.
Figures
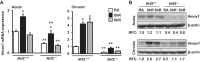
Similar articles
-
Nrf2 mediates hypoxia-inducible HIF1α activation in kidney tubular epithelial cells.Am J Physiol Renal Physiol. 2021 Mar 1;320(3):F464-F474. doi: 10.1152/ajprenal.00501.2020. Epub 2021 Jan 25. Am J Physiol Renal Physiol. 2021. PMID: 33491566 Free PMC article.
-
Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling.Arch Biochem Biophys. 2010 Sep 1;501(1):142-50. doi: 10.1016/j.abb.2010.06.011. Epub 2010 Jun 15. Arch Biochem Biophys. 2010. PMID: 20558128
-
Signal transduction involved in lipoxin A4‑induced protection of tubular epithelial cells against hypoxia/reoxygenation injury.Mol Med Rep. 2017 Apr;15(4):1682-1692. doi: 10.3892/mmr.2017.6195. Epub 2017 Feb 13. Mol Med Rep. 2017. PMID: 28259922 Free PMC article.
-
Roles of Nrf2 in Protecting the Kidney from Oxidative Damage.Int J Mol Sci. 2020 Apr 22;21(8):2951. doi: 10.3390/ijms21082951. Int J Mol Sci. 2020. PMID: 32331329 Free PMC article. Review.
-
Renal tubular cell death and inflammation response are regulated by the MAPK-ERK-CREB signaling pathway under hypoxia-reoxygenation injury.J Recept Signal Transduct Res. 2019 Oct-Dec;39(5-6):383-391. doi: 10.1080/10799893.2019.1698050. Epub 2019 Nov 29. J Recept Signal Transduct Res. 2019. PMID: 31782334 Review.
Cited by
-
Efflux Transporters Regulate Arsenite-Induced Genotoxicity in Double Negative and Double Positive T Cells.Toxicol Sci. 2017 Jul 1;158(1):127-139. doi: 10.1093/toxsci/kfx075. Toxicol Sci. 2017. PMID: 28472378 Free PMC article.
-
Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer.Exp Mol Med. 2024 Mar;56(3):501-514. doi: 10.1038/s12276-024-01180-8. Epub 2024 Mar 1. Exp Mol Med. 2024. PMID: 38424190 Free PMC article. Review.
-
Molecular Regulation of Heme Oxygenase-1 Expression by E2F Transcription Factor 2 in Lung Fibroblast Cells: Relevance to Idiopathic Pulmonary Fibrosis.Biomolecules. 2022 Oct 21;12(10):1531. doi: 10.3390/biom12101531. Biomolecules. 2022. PMID: 36291740 Free PMC article.
-
Suppression of NRF2 Activity by HIF-1α Promotes Fibrosis after Ischemic Acute Kidney Injury.Antioxidants (Basel). 2022 Sep 14;11(9):1810. doi: 10.3390/antiox11091810. Antioxidants (Basel). 2022. PMID: 36139884 Free PMC article.
-
Zebrafish, a Novel Model System to Study Uremic Toxins: The Case for the Sulfur Amino Acid Lanthionine.Int J Mol Sci. 2018 Apr 29;19(5):1323. doi: 10.3390/ijms19051323. Int J Mol Sci. 2018. PMID: 29710830 Free PMC article.
References
-
- Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11: 965–973, 2000. - PubMed
-
- Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 36: 166–174, 2007. - PubMed
-
- Bell CL, Tenenhouse HS, Scriver CR. Initiation and characterization of primary mouse kidney epithelial cultures. In Vitro Cell Dev Biol 24: 683–695, 1988. - PubMed
-
- Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 28: 1485–1490, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

