Plasmodium Helical Interspersed Subtelomeric (PHIST) Proteins, at the Center of Host Cell Remodeling
- PMID: 27582258
- PMCID: PMC5116875
- DOI: 10.1128/MMBR.00014-16
Plasmodium Helical Interspersed Subtelomeric (PHIST) Proteins, at the Center of Host Cell Remodeling
Abstract
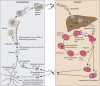
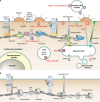
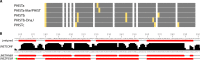
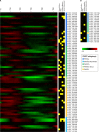
During the asexual cycle, Plasmodium falciparum extensively remodels the human erythrocyte to make it a suitable host cell. A large number of exported proteins facilitate this remodeling process, which causes erythrocytes to become more rigid, cytoadherent, and permeable for nutrients and metabolic products. Among the exported proteins, a family of 89 proteins, called the Plasmodium helical interspersed subtelomeric (PHIST) protein family, has been identified. While also found in other Plasmodium species, the PHIST family is greatly expanded in P. falciparum. Although a decade has passed since their first description, to date, most PHIST proteins remain uncharacterized and are of unknown function and localization within the host cell, and there are few data on their interactions with other host or parasite proteins. However, over the past few years, PHIST proteins have been mentioned in the literature at an increasing rate owing to their presence at various localizations within the infected erythrocyte. Expression of PHIST proteins has been implicated in molecular and cellular processes such as the surface display of PfEMP1, gametocytogenesis, changes in cell rigidity, and also cerebral and pregnancy-associated malaria. Thus, we conclude that PHIST proteins are central to host cell remodeling, but despite their obvious importance in pathology, PHIST proteins seem to be understudied. Here we review current knowledge, shed light on the definition of PHIST proteins, and discuss these proteins with respect to their localization and probable function. We take into consideration interaction studies, microarray analyses, or data from blood samples from naturally infected patients to combine all available information on this protein family.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Plasmodium helical interspersed subtelomeric family-an enigmatic piece of the Plasmodium biology puzzle.Parasitol Res. 2019 Oct;118(10):2753-2766. doi: 10.1007/s00436-019-06420-9. Epub 2019 Aug 15. Parasitol Res. 2019. PMID: 31418110 Review.
-
A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface.FASEB J. 2014 Oct;28(10):4420-33. doi: 10.1096/fj.14-256057. Epub 2014 Jun 30. FASEB J. 2014. PMID: 24983468 Free PMC article.
-
Plasmodium falciparum Plasmodium helical interspersed subtelomeric proteins contribute to cytoadherence and anchor P. falciparum erythrocyte membrane protein 1 to the host cell cytoskeleton.Cell Microbiol. 2016 Oct;18(10):1415-28. doi: 10.1111/cmi.12583. Epub 2016 Apr 26. Cell Microbiol. 2016. PMID: 26916885 Free PMC article.
-
PHISTc protein family members localize to different subcellular organelles and bind Plasmodium falciparum major virulence factor PfEMP-1.FEBS J. 2018 Jan;285(2):294-312. doi: 10.1111/febs.14340. Epub 2017 Dec 10. FEBS J. 2018. PMID: 29155505
-
Remodeling of human red cells infected with Plasmodium falciparum and the impact of PHIST proteins.Blood Cells Mol Dis. 2013 Oct;51(3):195-202. doi: 10.1016/j.bcmd.2013.06.003. Epub 2013 Jul 21. Blood Cells Mol Dis. 2013. PMID: 23880461 Review.
Cited by
-
Interaction Analysis of a Plasmodium falciparum PHISTa-like Protein and PfEMP1 Proteins.Front Microbiol. 2020 Nov 13;11:611190. doi: 10.3389/fmicb.2020.611190. eCollection 2020. Front Microbiol. 2020. PMID: 33281807 Free PMC article.
-
Machine learning for artemisinin resistance in malaria treatment across in vivo-in vitro platforms.iScience. 2022 Feb 10;25(3):103910. doi: 10.1016/j.isci.2022.103910. eCollection 2022 Mar 18. iScience. 2022. PMID: 35243261 Free PMC article.
-
Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions.PLoS Pathog. 2017 Aug 14;13(8):e1006552. doi: 10.1371/journal.ppat.1006552. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28806784 Free PMC article.
-
PFI1785w: A highly conserved protein associated with pregnancy associated malaria.PLoS One. 2017 Nov 9;12(11):e0187817. doi: 10.1371/journal.pone.0187817. eCollection 2017. PLoS One. 2017. PMID: 29121643 Free PMC article.
-
Comparative analysis of codon usage patterns of Plasmodium helical interspersed subtelomeric (PHIST) proteins.Front Microbiol. 2023 Dec 14;14:1320060. doi: 10.3389/fmicb.2023.1320060. eCollection 2023. Front Microbiol. 2023. PMID: 38156001 Free PMC article.
References
-
- WHO. 2015. World malaria report 2015. WHO, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

