Key regulators of galectin-glycan interactions
- PMID: 27582340
- PMCID: PMC5832938
- DOI: 10.1002/pmic.201600116
Key regulators of galectin-glycan interactions
Abstract
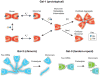
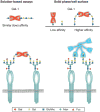
Protein-ligand interactions serve as fundamental regulators of numerous biological processes. Among protein-ligand pairs, glycan binding proteins (GBPs) and the glycans they recognize represent unique and highly complex interactions implicated in a broad range of regulatory activities. With few exceptions, cell surface receptors and secreted proteins are heavily glycosylated. As these glycans often represent highly regulatable post-translational modifications, alterations in glycosylation can fundamentally impact GBP recognition. Among GBPs, galectins in particular appear to engage a diverse set of glycan determinants to impact a broad range of biological processes. In this review, we will explore factors that impact galectin activity, including the effect of glycan modification on galectin-glycan interactions.
Keywords: Frontal Affinity Chromatography; Galectin; Glycan; Glycan Binding Protein; Glycoproteomics; Surface Plasmon Resonance.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
-
- Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5:1087–1104. - PubMed
-
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. - PubMed
-
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

