Platelet and Erythrocyte Sources of S1P Are Redundant for Vascular Development and Homeostasis, but Both Rendered Essential After Plasma S1P Depletion in Anaphylactic Shock
- PMID: 27582371
- PMCID: PMC5064286
- DOI: 10.1161/CIRCRESAHA.116.308929
Platelet and Erythrocyte Sources of S1P Are Redundant for Vascular Development and Homeostasis, but Both Rendered Essential After Plasma S1P Depletion in Anaphylactic Shock
Abstract
Rationale: Sphingosine-1-phosphate (S1P) signaling is essential for vascular development and postnatal vascular homeostasis. The relative importance of S1P sources sustaining these processes remains unclear.
Objective: To address the level of redundancy in bioactive S1P provision to the developing and mature vasculature.
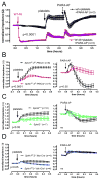
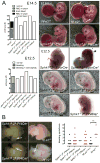
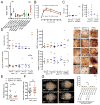
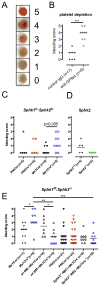
Methods and results: S1P production was selectively impaired in mouse platelets, erythrocytes, endothelium, or smooth muscle cells by targeted deletion of genes encoding sphingosine kinases -1 and -2. S1P deficiency impaired aggregation and spreading of washed platelets and profoundly reduced their capacity to promote endothelial barrier function ex vivo. However, and in contrast to recent reports, neither platelets nor any other source of S1P was essential for vascular development, vascular integrity, or hemostasis/thrombosis. Yet rapid and profound depletion of plasma S1P during systemic anaphylaxis rendered both platelet- and erythrocyte-derived S1P essential for survival, with a contribution from blood endothelium observed only in the absence of circulating sources. Recovery was sensitive to aspirin in mice with but not without platelet S1P, suggesting that platelet activation and stimulus-response coupling is needed. S1P deficiency aggravated vasoplegia in this model, arguing a vital role for S1P in maintaining vascular resistance during recovery from circulatory shock. Accordingly, the S1P2 receptor mediated most of the survival benefit of S1P, whereas the endothelial S1P1 receptor was dispensable for survival despite its importance for maintaining vascular integrity.
Conclusions: Although source redundancy normally secures essential S1P signaling in developing and mature blood vessels, profound depletion of plasma S1P renders both erythrocyte and platelet S1P pools necessary for recovery and high basal plasma S1P levels protective during anaphylactic shock.
Keywords: anaphylaxis; endothelium; shock; sphingosine-1-phosphate; vascular endothelial function; vascular permeability; vascular tone regulation.
© 2016 American Heart Association, Inc.
Figures




References
-
- Spiegel S, Milstien S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

