Infective endocarditis
- PMID: 27582414
- PMCID: PMC5240923
- DOI: 10.1038/nrdp.2016.59
Infective endocarditis
Abstract
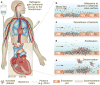
Infective endocarditis (IE) is a rare, life-threatening disease that has long-lasting effects even among patients who survive and are cured. IE disproportionately affects those with underlying structural heart disease and is increasingly associated with health care contact, particularly in patients who have intravascular prosthetic material. In the setting of bacteraemia with a pathogenic organism, an infected vegetation may form as the end result of complex interactions between invading microorganisms and the host immune system. Once established, IE can involve almost any organ system in the body. The diagnosis of IE may be difficult to establish and a strategy that combines clinical, microbiological and echocardiography results has been codified in the modified Duke criteria. In cases of blood culture-negative IE, the diagnosis may be especially challenging, and novel microbiological and imaging techniques have been developed to establish its presence. Once diagnosed, IE is best managed by a multidisciplinary team with expertise in infectious diseases, cardiology and cardiac surgery. Antibiotic prophylaxis for the prevention of IE remains controversial. Efforts to develop a vaccine that targets common bacterial causes of IE are ongoing, but have not yet yielded a commercially available product.
Conflict of interest statement
V.G.F. reports the following potential conflicts of interest: Chair of the Scientific Advisory Board for Merck V710 Staphylococcus aureus vaccine; paid consultant for Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea and Affinergy; grants pending from MedImmune, Actavis/Forest/Cerexa, Pfizer, Merck/Cubist, Advanced Liquid Logics, Theravance and Novartis; royalties from UpToDate; personal fees for development or presentation of educational presentations from Green Cross, Cubist, Cerexa, Durata and Theravance,; and a patent pending related to sepsis diagnostics. T.L.H. reports the following potential conflicts of interest: paid consultant for The Medicines Company and Basilea Pharmaceutica; and royalties from UpToDate. A.S.B. reports the following potential conflicts of interest: Research Grants from ContraFect and Theravance; and Advisory Board member for ContraFect. J.M.M. reports the following potential conflicts of interest: consulting honoraria and/or research grants from AbbVie, Bristol-Myers Squibb, Cubist, Genentech, Merck, Novartis, Gilead Sciences and ViiV Healthcare. The other authors declare no potential conflicts of interest.
Figures



References
-
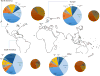
- Bin Abdulhak AA, et al. Global and regional burden of infective endocarditis, 1990–2010: a systematic review of the literature. Glob Heart. 2014;9:131–143. - PubMed
-
- Murdoch DR, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Archives of internal medicine. 2009;169:463–473. This prospective cohort study of 2781 adults with definite endocarditis demonstrated that IE had shifted from a subacute disease of younger people with rheumatic valvular abnormalities, to one in which the presentation is more acute and is characterized by a high rate of S. aureus infection in patients with previous health care exposure. - PMC - PubMed
-
- Thayer W. Studies on bacterial (infective) endocarditis. Johns Hopkins Hosp Rep. 1926;22:1.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

