The Second Spiking Threshold: Dynamics of Laminar Network Spiking in the Visual Cortex
- PMID: 27582693
- PMCID: PMC4987378
- DOI: 10.3389/fnsys.2016.00065
The Second Spiking Threshold: Dynamics of Laminar Network Spiking in the Visual Cortex
Abstract
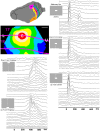
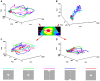
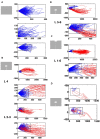
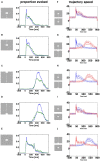
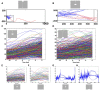
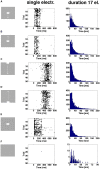
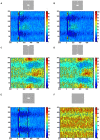
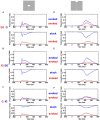
Most neurons have a threshold separating the silent non-spiking state and the state of producing temporal sequences of spikes. But neurons in vivo also have a second threshold, found recently in granular layer neurons of the primary visual cortex, separating spontaneous ongoing spiking from visually evoked spiking driven by sharp transients. Here we examine whether this second threshold exists outside the granular layer and examine details of transitions between spiking states in ferrets exposed to moving objects. We found the second threshold, separating spiking states evoked by stationary and moving visual stimuli from the spontaneous ongoing spiking state, in all layers and zones of areas 17 and 18 indicating that the second threshold is a property of the network. Spontaneous and evoked spiking, thus can easily be distinguished. In addition, the trajectories of spontaneous ongoing states were slow, frequently changing direction. In single trials, sharp as well as smooth and slow transients transform the trajectories to be outward directed, fast and crossing the threshold to become evoked. Although the speeds of the evolution of the evoked states differ, the same domain of the state space is explored indicating uniformity of the evoked states. All evoked states return to the spontaneous evoked spiking state as in a typical mono-stable dynamical system. In single trials, neither the original spiking rates, nor the temporal evolution in state space could distinguish simple visual scenes.
Keywords: dynamical systems; evoked activity; object vision; spontaneous activity; visual motion.
Figures


Similar articles
-
Breaking the Excitation-Inhibition Balance Makes the Cortical Network's Space-Time Dynamics Distinguish Simple Visual Scenes.Front Syst Neurosci. 2017 Mar 20;11:14. doi: 10.3389/fnsys.2017.00014. eCollection 2017. Front Syst Neurosci. 2017. PMID: 28377701 Free PMC article.
-
Visually Evoked Spiking Evolves While Spontaneous Ongoing Dynamics Persist.Front Syst Neurosci. 2016 Jan 8;9:183. doi: 10.3389/fnsys.2015.00183. eCollection 2015. Front Syst Neurosci. 2016. PMID: 26778982 Free PMC article.
-
Network activity influences the subthreshold and spiking visual responses of pyramidal neurons in the three-layer turtle cortex.J Neurophysiol. 2017 Oct 1;118(4):2142-2155. doi: 10.1152/jn.00340.2017. Epub 2017 Jul 26. J Neurophysiol. 2017. PMID: 28747466 Free PMC article.
-
Space-Time Dynamics of Membrane Currents Evolve to Shape Excitation, Spiking, and Inhibition in the Cortex at Small and Large Scales.Neuron. 2017 Jun 7;94(5):934-942. doi: 10.1016/j.neuron.2017.04.038. Neuron. 2017. PMID: 28595049 Review.
-
Non-linear dynamics of columns of cat visual cortex revealed by simulation and experiment.Ciba Found Symp. 1994;184:88-99; discussion 99-103, 120-8. doi: 10.1002/9780470514610.ch5. Ciba Found Symp. 1994. PMID: 7882763 Review.
Cited by
-
Local networks from different parts of the human cerebral cortex generate and share the same population dynamic.Cereb Cortex Commun. 2022 Oct 28;3(4):tgac040. doi: 10.1093/texcom/tgac040. eCollection 2022. Cereb Cortex Commun. 2022. PMID: 36530950 Free PMC article.
-
Neural Manifolds for the Control of Movement.Neuron. 2017 Jun 7;94(5):978-984. doi: 10.1016/j.neuron.2017.05.025. Neuron. 2017. PMID: 28595054 Free PMC article. Review.
-
Breaking the Excitation-Inhibition Balance Makes the Cortical Network's Space-Time Dynamics Distinguish Simple Visual Scenes.Front Syst Neurosci. 2017 Mar 20;11:14. doi: 10.3389/fnsys.2017.00014. eCollection 2017. Front Syst Neurosci. 2017. PMID: 28377701 Free PMC article.
References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate- a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Methodol. 57, 289–300.
-
- Douglas R., Martin K. A. C., Whitteridge D. (1989). A canonical microcircuit for neocortex. Neural Comput. 1, 480–488. 10.1162/neco.1989.1.4.480 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

