Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species
- PMID: 27582729
- PMCID: PMC4988121
- DOI: 10.3389/fmicb.2016.01230
Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species
Abstract
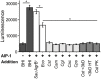
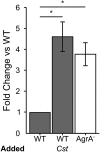
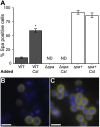
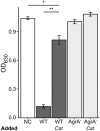
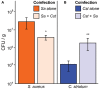
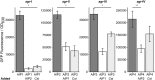
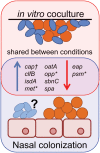
Staphylococcus aureus-human interactions result in a continuum of outcomes from commensalism to pathogenesis. S. aureus is a clinically important pathogen that asymptomatically colonizes ~25% of humans as a member of the nostril and skin microbiota, where it resides with other bacteria including commensal Corynebacterium species. Commensal Corynebacterium spp. are also positively correlated with S. aureus in chronic polymicrobial diabetic foot infections, distinct from acute monomicrobial S. aureus infections. Recent work by our lab and others indicates that microbe-microbe interactions between S. aureus and human skin/nasal commensals, including Corynebacterium species, affect S. aureus behavior and fitness. Thus, we hypothesized that S. aureus interactions with Corynebacterium spp. diminish S. aureus virulence. We tested this by assaying for changes in S. aureus gene expression during in vitro mono- versus coculture with Corynebacterium striatum, a common skin and nasal commensal. We observed a broad shift in S. aureus gene transcription during in vitro growth with C. striatum, including increased transcription of genes known to exhibit increased expression during human nasal colonization and decreased transcription of virulence genes. S. aureus uses several regulatory pathways to transition between commensal and pathogenic states. One of these, the quorum signal accessory gene regulator (agr) system, was strongly inhibited in response to Corynebacterium spp. Phenotypically, S. aureus exposed to C. striatum exhibited increased adhesion to epithelial cells, reflecting a commensal state, and decreased hemolysin activity, reflecting an attenuation of virulence. Consistent with this, S. aureus displayed diminished fitness in experimental in vivo coinfection with C. striatum when compared to monoinfection. These data support a model in which S. aureus shifts from virulence toward a commensal state when exposed to commensal Corynebacterium species.
Keywords: Corynebacterium; Staphylococcus aureus; agr system; commensal bacteria; microbiome; quorum sensing (QS).
Figures
References
-
- Abe S., Takayama K.-I., Kinoshita S. (1967). Taxonomical studies on glutamic acid-producing bacteria. J. Gen. Appl. Microbiol. 13 279–301. 10.2323/jgam.13.279 - DOI
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J., Smith J. A., et al. (2002). Short Protocols in Molecular Biology: A Compendium of Methods from Current Protocols in Molecular Biology. New York, NY: Wiley.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

