Clinical Outcomes and Women's Experiences before and after the Introduction of Mifepristone into Second-Trimester Medical Abortion Services in South Africa
- PMID: 27583448
- PMCID: PMC5008795
- DOI: 10.1371/journal.pone.0161843
Clinical Outcomes and Women's Experiences before and after the Introduction of Mifepristone into Second-Trimester Medical Abortion Services in South Africa
Abstract
Objective: To document clinical outcomes and women's experiences following the introduction of mifepristone into South African public sector second-trimester medical abortion services, and compare with historic cohorts receiving misoprostol-only.
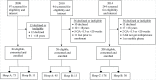
Methods: Repeated cross-sectional observational studies documented service delivery and experiences of women undergoing second-trimester medical abortion in public sector hospitals in the Western Cape, South Africa. Women recruited to the study in 2008 (n = 84) and 2010 (n = 58) received misoprostol only. Those recruited in 2014 (n = 208) received mifepristone and misoprostol. Consenting women were interviewed during hospitalization by study fieldworkers with respect to socio-demographic information, reproductive history, and their experiences with the abortion. Clinical details were extracted from medical charts following discharge. Telephone follow-up interviews to record delayed complications were conducted 2-4 weeks after discharge for the 2014 cohort.
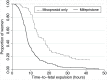
Results: The 2014 cohort received 200 mg mifepristone, which was self-administered 24-48 hours prior to admission. For all cohorts, following hospital admission, initial misoprostol doses were generally administered vaginally: 800 mcg in the 2014 cohort and 600 mcg in the earlier cohorts. Women received subsequent doses of misoprostol 400 mcg orally every 3-4 hours until fetal expulsion. Thereafter, uterine evacuation of placental tissue was performed as needed. With one exception, all women in all cohorts expelled the fetus. Median time-to-fetal expulsion was reduced to 8.0 hours from 14.5 hours (p<0.001) in the mifepristone compared to the 2010 misoprostol-only cohort (time of fetal expulsion was not recorded in 2008). Uterine evacuation of placental tissue using curettage or vacuum aspiration was more often performed (76% vs. 58%, p<0.001) for those receiving mifepristone; major complication rates were unchanged. Hospitalization duration and extreme pain levels were reduced (p<0.001), but side effects of medication were similar or more common for the mifepristone cohort. Overall satisfaction remained unchanged (95% vs. 91%), while other acceptability measures were higher (p<0.001) for the mifepristone compared to the misoprostol-only cohorts.
Conclusion: The introduction of a combined mifepristone-misoprostol regimen into public sector second-trimester medical abortion services in South Africa has been successful with shorter time-to-abortion events, less extreme pain and greater acceptability for women. High rates of uterine evacuation for placental tissue need to be addressed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Simultaneous Administration Compared With a 24-Hour Mifepristone-Misoprostol Interval in Second-Trimester Abortion: A Randomized Controlled Trial.Obstet Gynecol. 2016 Nov;128(5):1077-1083. doi: 10.1097/AOG.0000000000001688. Obstet Gynecol. 2016. PMID: 27741182 Clinical Trial.
-
Second trimester medical abortion with mifepristone followed by unlimited dosing of buccal misoprostol in Armenia.Eur J Contracept Reprod Health Care. 2017 Feb;22(1):76-80. doi: 10.1080/13625187.2016.1258461. Epub 2016 Nov 21. Eur J Contracept Reprod Health Care. 2017. PMID: 27871191
-
Introducing medication abortion into public sector facilities in KwaZulu-Natal, South Africa: an operations research study.Contraception. 2015 Oct;92(4):330-8. doi: 10.1016/j.contraception.2015.07.001. Epub 2015 Jul 7. Contraception. 2015. PMID: 26162575
-
Second trimester medical abortion with mifepristone-misoprostol and misoprostol alone: a review of methods and management.Reprod Health Matters. 2008 May;16(31 Suppl):162-72. doi: 10.1016/S0968-8080(08)31371-8. Reprod Health Matters. 2008. PMID: 18772097 Review.
-
Expulsion at home for early medical abortion: A systematic review with meta-analyses.Acta Obstet Gynecol Scand. 2021 Apr;100(4):727-735. doi: 10.1111/aogs.14025. Epub 2020 Nov 28. Acta Obstet Gynecol Scand. 2021. PMID: 33063314
Cited by
-
The efficacy of mifepristone-misoprostol regimen versus misoprostol-only for medication abortion at 22 + 0/7 to 30 + 0/7 weeks' gestation.Arch Gynecol Obstet. 2025 Mar;311(3):749-756. doi: 10.1007/s00404-024-07737-2. Epub 2024 Sep 18. Arch Gynecol Obstet. 2025. PMID: 39292225 Free PMC article.
-
Outpatient medical management of later second trimester abortion (18-23.6 weeks) with procedural evacuation backup: A large case series.Contracept X. 2024 Feb 17;6:100104. doi: 10.1016/j.conx.2024.100104. eCollection 2024. Contracept X. 2024. PMID: 38515629 Free PMC article.
-
Nego-feminism as a strategy to improve access to abortion in sub-saharan Africa.Reprod Health. 2024 Nov 28;21(1):175. doi: 10.1186/s12978-024-01914-x. Reprod Health. 2024. PMID: 39605066 Free PMC article.
-
The costs and cost effectiveness of providing second-trimester medical and surgical safe abortion services in Western Cape Province, South Africa.PLoS One. 2018 Jun 28;13(6):e0197485. doi: 10.1371/journal.pone.0197485. eCollection 2018. PLoS One. 2018. PMID: 29953434 Free PMC article.
References
-
- Choice on Termination of Pregnancy, Act No.92 of 1996. Government Gazette, State Paper of the Republic of South Africa, (1996).
-
- Sinjani/TOP’s. 2014–2015 financial year. Western Cape Government: Health. [Online]. Available at: https://sinjani.pgwc.gov.za/live/sinjani (Restricted access). Western Cape Department of Health, South Africa. 2014.
-
- Berer M. A Critical Appraisal of Laws on Second Trimester Abortion. Reprod Health Matters. 2008;16(31, Suppl):3–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical