Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: A meta-analysis of randomized clinical trials
- PMID: 27583876
- PMCID: PMC5008560
- DOI: 10.1097/MD.0000000000004611
Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: A meta-analysis of randomized clinical trials
Abstract
Background: Anti-PD-1/PD-L1 antibody therapy is a promising clinical treatment for nonsmall-cell lung cancer (NSCLC). However, whether anti-PD-1/PD-L1 antibody therapy can provide added benefits for heavily pretreated patients with advanced NSCLC and whether the efficacy of anti-PD-1/PD-L1 antibody therapy relates to the tumor PD-L1 expression level remain controversial. Thus, this meta-analysis evaluated the efficacy and safety of anti-PD-1/PD-L1 antibody therapy for pretreated patients with advanced NSCLC.
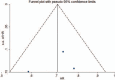
Methods: Randomized clinical trials were retrieved by searching the PubMed, EMBASE, ASCO meeting abstract, clinicaltrial.gov, and Cochrane library databases. The pooled hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS), and odds ratios for the overall response rate and adverse events (AEs) were calculated by STATA software.
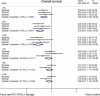
Results: Three randomized clinical trials involving 1141 pretreated patients with advanced NSCLC were included. These trials all compared the efficacy and safety of anti-PD-1/PD-L1 antibodies (nivolumab and MPDL3280A) with docetaxel. The results suggested that, for all patients, anti-PD-1/PD-L1 therapy could acquire a greater overall response (odds ratio = 1.50, 95% CI: 1.08-2.07, P = 0.015, P for heterogeneity [Ph] = 0.620) and longer OS (HR = 0.71, 95% CI: 0.61-0.81, P < 0.001, Ph = 0.361) than docetaxel, but not PFS (HR = 0.83, 95% CI: 0.65-1.06, P = 0.134; Ph = 0.031). Subgroup analyses according to the tumor PD-L1 expression level showed that anti-PD-1/PD-L1 therapy could significantly improve both OS and PFS in patients with high expressions of PD-L1, but not in those with low expressions. Generally, the rates of grade 3 or 4 AEs of anti-PD-1/PD-L1 therapy were significantly lower than that of docetaxel. However, the risks of pneumonitis and hypothyroidism were significantly higher.
Conclusion: Anti-PD-1/PD-L1 antibody therapy may significantly improve the outcomes for pretreated advanced NSCLC patients, with a better safety profile than docetaxel.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures







Similar articles
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
A meta-analysis of efficacy and safety of antibodies targeting PD-1/PD-L1 in treatment of advanced nonsmall cell lung cancer.Medicine (Baltimore). 2016 Dec;95(52):e5539. doi: 10.1097/MD.0000000000005539. Medicine (Baltimore). 2016. PMID: 28033249 Free PMC article. Review.
-
Anti-PD-1/PD-L1 Antibody Therapy for Pretreated Advanced or Metastatic Nonsmall Cell Lung Carcinomas and the Correlation between PD-L1 Expression and Treatment Effectiveness: An Update Meta-Analysis of Randomized Clinical Trials.Biomed Res Int. 2018 Sep 24;2018:3820956. doi: 10.1155/2018/3820956. eCollection 2018. Biomed Res Int. 2018. PMID: 30345301 Free PMC article.
-
Comparative efficacy and safety of PD-1/PD-L1 Inhibitors versus platinum-based chemotherapy for the first-line treatment of advanced non-small cell lung cancer: a meta analysis of randomized controlled trials.Pharmacol Res. 2020 Oct;160:105194. doi: 10.1016/j.phrs.2020.105194. Epub 2020 Sep 13. Pharmacol Res. 2020. PMID: 32937178
Cited by
-
Immune Checkpoint Immunotherapy for Non-Small Cell Lung Cancer: Benefits and Pulmonary Toxicities.Chest. 2018 Dec;154(6):1416-1423. doi: 10.1016/j.chest.2018.08.1048. Epub 2018 Sep 4. Chest. 2018. PMID: 30189190 Free PMC article. Review.
-
Effective nivolumab sequential thoracic radiotherapy in elderly patients with advanced squamous cell lung cancer: did radiation therapy play a role? A case report.Onco Targets Ther. 2018 Aug 7;11:4621-4629. doi: 10.2147/OTT.S176226. eCollection 2018. Onco Targets Ther. 2018. PMID: 30122953 Free PMC article.
-
Retrospective observational cohort study on innovation in oncology and progress in survival: How far have we gotten in the two decades of treating patients with advanced non-small cell lung cancer as a single population?PLoS One. 2020 May 12;15(5):e0232669. doi: 10.1371/journal.pone.0232669. eCollection 2020. PLoS One. 2020. PMID: 32396541 Free PMC article.
-
Artificial Intelligence Tools for Refining Lung Cancer Screening.J Clin Med. 2020 Nov 27;9(12):3860. doi: 10.3390/jcm9123860. J Clin Med. 2020. PMID: 33261057 Free PMC article. Review.
-
Resistance in Lung Cancer Immunotherapy and How to Overcome It: Insights from the Genetics Perspective and Combination Therapies Approach.Cells. 2025 Apr 12;14(8):587. doi: 10.3390/cells14080587. Cells. 2025. PMID: 40277912 Free PMC article. Review.
References
-
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. - PubMed
-
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000; 18:2354–2362. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18:2095–2103. - PubMed
-
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22:1589–1597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

