Phosphatidylserine and Phosphatidylethanolamine Bind to Protein Z Cooperatively and with Equal Affinity
- PMID: 27584039
- PMCID: PMC5008636
- DOI: 10.1371/journal.pone.0161896
Phosphatidylserine and Phosphatidylethanolamine Bind to Protein Z Cooperatively and with Equal Affinity
Abstract
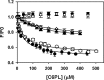
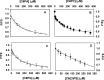
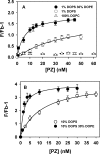
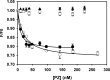
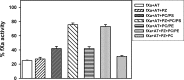
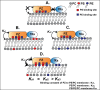
Protein Z (PZ) is an anticoagulant that binds with high affinity to Protein Z-dependent protease inhibitor (ZPI) and accelerates the rate of ZPI-mediated inhibition of factor Xa (fXa) by more than 1000-fold in the presence of Ca2+ and phospholipids. PZ promotion of the ZPI-fXa interaction results from the anchoring of the Gla domain of PZ onto phospholipid surfaces and positioning the bound ZPI in close proximity to the Gla-anchored fXa, forming a ternary complex of PZ/ZPI/fXa. Although interaction of PZ with phospholipid membrane appears to be absolutely crucial for its cofactor activity, little is known about the binding of different phospholipids to PZ. The present study was conceived to understand the interaction of different phospholipids with PZ. Experiments with both soluble lipids and model membranes revealed that PZ binds to phosphatidylserine (PS) and phosphatidylethanolamine (PE) with equal affinity (Kd~48 μM); further, PS and PE bound to PZ synergistically. Equilibrium dialysis experiments revealed two lipid-binding sites for both PS and PE. PZ binds with weaker affinity to other phospholipids, e.g., phosphatidic acid, phosphatidylglycerol, phosphatidylcholine and binding of these lipids is not synergistic with respect to PS. Both PS and PE -containing membranes supported the formation of a fXa-PZ complex. PZ protection of fXa from antithrombin inhibition were also shown to be comparable in presence of both PS: PC and PE: PC membranes. These findings are particularly important and intriguing since they suggest a special affinity of PZ, in vivo, towards activated platelets, the primary membrane involved in blood coagulation process.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Protein Z-dependent protease inhibitor binds to the C-terminal domain of protein Z.J Biol Chem. 2008 Jul 18;283(29):19922-6. doi: 10.1074/jbc.M802639200. Epub 2008 May 23. J Biol Chem. 2008. PMID: 18502758 Free PMC article.
-
Basis for the specificity and activation of the serpin protein Z-dependent proteinase inhibitor (ZPI) as an inhibitor of membrane-associated factor Xa.J Biol Chem. 2010 Jun 25;285(26):20399-409. doi: 10.1074/jbc.M110.112748. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427285 Free PMC article.
-
Lipid oxidation inactivates the anticoagulant function of protein Z-dependent protease inhibitor (ZPI).J Biol Chem. 2017 Sep 1;292(35):14625-14635. doi: 10.1074/jbc.M117.793901. Epub 2017 Jul 17. J Biol Chem. 2017. PMID: 28717005 Free PMC article.
-
Protein Z-dependent regulation of coagulation.Thromb Haemost. 2001 Jul;86(1):8-13. Thromb Haemost. 2001. PMID: 11487045 Review.
-
Protein Z, an anticoagulant protein with expanding role in reproductive biology.Reproduction. 2013 Jun 27;146(2):R73-80. doi: 10.1530/REP-13-0072. Print 2013 Aug. Reproduction. 2013. PMID: 23690629 Review.
Cited by
-
Diacylation of Peptides Enables the Construction of Functional Vesicles for Drug-Carrying Liposomes.Angew Chem Int Ed Engl. 2025 May 12;64(20):e202421932. doi: 10.1002/anie.202421932. Epub 2025 Apr 7. Angew Chem Int Ed Engl. 2025. PMID: 39776211
-
Phosphatidylserine and phosphatidylethanolamine regulate the structure and function of FVIIa and its interaction with soluble tissue factor.Biosci Rep. 2021 Feb 26;41(2):BSR20204077. doi: 10.1042/BSR20204077. Biosci Rep. 2021. PMID: 33479740 Free PMC article.
-
Small molecule modulation of protein corona for deep plasma proteome profiling.Nat Commun. 2024 Nov 7;15(1):9638. doi: 10.1038/s41467-024-53966-z. Nat Commun. 2024. PMID: 39511193 Free PMC article.
-
Deep Plasma Proteome Profiling by Modulating Single Nanoparticle Protein Corona with Small Molecules.bioRxiv [Preprint]. 2024 Sep 9:2024.03.06.582595. doi: 10.1101/2024.03.06.582595. bioRxiv. 2024. PMID: 38496642 Free PMC article. Preprint.
-
Hepatitis C Virus p7 Induces Membrane Permeabilization by Interacting with Phosphatidylserine.Int J Mol Sci. 2020 Jan 30;21(3):897. doi: 10.3390/ijms21030897. Int J Mol Sci. 2020. PMID: 32019133 Free PMC article.
References
-
- Okajima K. Regulation of inflammatory responses by natural anticoagulants. Immunol Rev. 2001;184: 258–74. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

