Targeting proprotein convertases in furin-rich lung cancer cells results in decreased in vitro and in vivo growth
- PMID: 27584082
- PMCID: PMC6166887
- DOI: 10.1002/mc.22550
Targeting proprotein convertases in furin-rich lung cancer cells results in decreased in vitro and in vivo growth
Abstract
Proprotein convertases (PCs) are serine proteases with an active role in the post-translational processing of numerous inactive proteins to active proteins including many substrates of paramount importance in cancer development and progression. Furin (PCSKC3), a well-studied member of this family, is overexpressed in numerous human and experimental malignancies. In the present communication, we treated two furin-overexpressing non-small cell carcinoma (NSCLC) cell lines (Calu-6 and HOP-62) with the PC inhibitor CMK (Decanoyl-Arg-Val-Lys-Arg-chloromethylketone). This resulted in a diminished IGF-1R processing and a simultaneous decrease in cell proliferation of two NSCLC lines. Similarly, growth of subcutaneous xenografts of both cell lines, were partially inhibited by an in vivo treatment with the same drug. These observations point to a potential role of PC inhibitors in cancer therapy. © 2016 Wiley Periodicals, Inc.
Keywords: IGF-1R; PCSKC3; furin; lung cancer; proprotein convertases; tumor development.
© 2016 Wiley Periodicals, Inc.
Figures
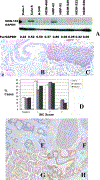


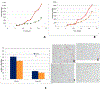
References
-
- Bassi DE, Fu J, Lopez de Cicco R and Klein-Szanto AJP Proprotein convertases: “Master Switches” in the regulation of tumor growth and progression. Molec. Carcinogenesis 2005; 44:151–161. - PubMed
-
- Scamuffa N, Calvo F, Chretien M, et al. Proprotein convertases: lessons from knockouts. Faseb J 2006; 20:1954–1963. - PubMed
-
- Taylor NA, Van De Ven WJ, and Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. Faseb J 2003; 17:1215–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

