Characterization of gprK Encoding a Putative Hybrid G-Protein-Coupled Receptor in Aspergillus fumigatus
- PMID: 27584150
- PMCID: PMC5008803
- DOI: 10.1371/journal.pone.0161312
Characterization of gprK Encoding a Putative Hybrid G-Protein-Coupled Receptor in Aspergillus fumigatus
Abstract
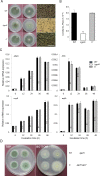
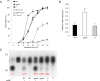
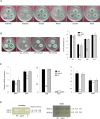
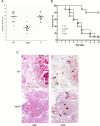
The G-protein-coupled receptor (GPCR) family represents the largest and most varied collection of membrane embedded proteins that are sensitized by ligand binding and interact with heterotrimeric G proteins. Despite their presumed critical roles in fungal biology, the functions of the GPCR family members in the opportunistic human pathogen Aspergillus fumigatus are largely unknown, as only two (GprC and GprD) of the 15 predicted GPCRs have been studied. Here, we characterize the gprK gene, which is predicted to encode a hybrid GPCR with both 7-transmembrane and regulator of G-protein signaling (RGS) domains. The deletion of gprK causes severely impaired asexual development coupled with reduced expression of key developmental activators. Moreover, ΔgprK results in hyper-activation of germination even in the absence of carbon source, and elevated expression and activity of the protein kinase A PkaC1. Furthermore, proliferation of the ΔgprK mutant is restricted on the medium when pentose is the sole carbon source, suggesting that GprK may function in external carbon source sensing. Notably, the absence of gprK results in reduced tolerance to oxidative stress and significantly lowered mRNA levels of the stress-response associated genes sakA and atfA. Activities of catalases and SODs are severely decreased in the ΔgprK mutant, indicating that GprK may function in proper activation of general stress response. The ΔgprK mutant is also defective in gliotoxin (GT) production and slightly less virulent toward the greater wax moth, Galleria mellonella. Transcriptomic studies reveal that a majority of transporters are down-regulated by ΔgprK. In summary, GprK is necessary for proper development, GT production, and oxidative stress response, and functions in down-regulating the PKA-germination pathway.
Conflict of interest statement
The authors have declared that no competing interests exist. Professor Jae-Hyuk Yu is an Academic Editor of PLOS ONE. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
RgsA Attenuates the PKA Signaling, Stress Response, and Virulence in the Human Opportunistic Pathogen Aspergillus fumigatus.Int J Mol Sci. 2019 Nov 11;20(22):5628. doi: 10.3390/ijms20225628. Int J Mol Sci. 2019. PMID: 31717953 Free PMC article.
-
Characteristics of a Regulator of G-Protein Signaling (RGS) rgsC in Aspergillus fumigatus.Front Microbiol. 2017 Oct 23;8:2058. doi: 10.3389/fmicb.2017.02058. eCollection 2017. Front Microbiol. 2017. PMID: 29109714 Free PMC article.
-
The Putative APSES Transcription Factor RgdA Governs Growth, Development, Toxigenesis, and Virulence in Aspergillus fumigatus.mSphere. 2020 Nov 11;5(6):e00998-20. doi: 10.1128/mSphere.00998-20. mSphere. 2020. PMID: 33177217 Free PMC article.
-
Genetic control of asexual development in aspergillus fumigatus.Adv Appl Microbiol. 2015;90:93-107. doi: 10.1016/bs.aambs.2014.09.004. Epub 2014 Nov 12. Adv Appl Microbiol. 2015. PMID: 25596030 Review.
-
Heterotrimeric G-protein signalers and RGSs in Aspergillus fumigatus.Pathogens. 2020 Oct 28;9(11):902. doi: 10.3390/pathogens9110902. Pathogens. 2020. PMID: 33126739 Free PMC article. Review.
Cited by
-
Aspergillus fumigatus G-Protein Coupled Receptors GprM and GprJ Are Important for the Regulation of the Cell Wall Integrity Pathway, Secondary Metabolite Production, and Virulence.mBio. 2020 Oct 13;11(5):e02458-20. doi: 10.1128/mBio.02458-20. mBio. 2020. PMID: 33051372 Free PMC article.
-
The Cell Wall Integrity Signaling Pathway and Its Involvement in Secondary Metabolite Production.J Fungi (Basel). 2017 Dec 6;3(4):68. doi: 10.3390/jof3040068. J Fungi (Basel). 2017. PMID: 29371582 Free PMC article. Review.
-
Overview of carbon and nitrogen catabolite metabolism in the virulence of human pathogenic fungi.Mol Microbiol. 2018 Feb;107(3):277-297. doi: 10.1111/mmi.13887. Epub 2017 Dec 29. Mol Microbiol. 2018. PMID: 29197127 Free PMC article. Review.
-
Comparative Characterization of G Protein α Subunits in Aspergillus fumigatus.Pathogens. 2020 Apr 9;9(4):272. doi: 10.3390/pathogens9040272. Pathogens. 2020. PMID: 32283604 Free PMC article.
-
RgsA Attenuates the PKA Signaling, Stress Response, and Virulence in the Human Opportunistic Pathogen Aspergillus fumigatus.Int J Mol Sci. 2019 Nov 11;20(22):5628. doi: 10.3390/ijms20225628. Int J Mol Sci. 2019. PMID: 31717953 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

