Kinsenoside screening with a microfluidic chip attenuates gouty arthritis through inactivating NF-κB signaling in macrophages and protecting endothelial cells
- PMID: 27584788
- PMCID: PMC5059859
- DOI: 10.1038/cddis.2016.255
Kinsenoside screening with a microfluidic chip attenuates gouty arthritis through inactivating NF-κB signaling in macrophages and protecting endothelial cells
Abstract
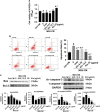
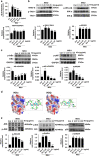
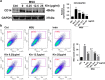
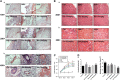
Gouty arthritis is a rheumatic disease that is characterized by the deposition of monosodium urate (MSU) in synovial joints cause by the increased serum hyperuricemia. This study used a three-dimensional (3D) flowing microfluidic chip to screen the effective candidate against MSU-stimulated human umbilical vein endothelial cell (HUVEC) damage, and found kinsenoside (Kin) to be the leading active component of Anoectochilus roxburghi, one of the Chinese medicinal plant widely used in the treatment of gouty arthritis clinically. Cell viability and apoptosis of HUVECs were evaluated, indicating that direct Kin stimulation and conditioned medium (CM) from Kin-treated macrophages both negatively modulated with MSU crystals. Additionally, Kin was capable of attenuating MSU-induced activation of nuclear factor-κB/mitogen-activated protein kinase (NF-κB/MAPK) signaling, targeting IκB kinase-α (IKKα) and IKKβ kinases of macrophages and influencing the expressions of NF-κB downstream cytokines and subsequent HUVEC bioactivity. Inflammasome NLR pyrin domain-containing 3 (NALP3) and toll-like receptor 2 (TLR2) were also inhibited after Kin treatment. Also, Kin downregulated CD14-mediated MSU crystals uptake in macrophages. In vivo study with MSU-injected ankle joints further revealed the significant suppression of inflammatory infiltration and endothelia impairment coupled with alleviation of ankle swelling and nociceptive response via Kin treatments. Taken together, these data implicated that Kin was the most effective candidate from Anoectochilus roxburghi to treat gouty arthritis clinically.
Figures




Similar articles
-
Curcumin ameliorates monosodium urate-induced gouty arthritis through Nod-like receptor 3 inflammasome mediation via inhibiting nuclear factor-kappa B signaling.J Cell Biochem. 2019 Apr;120(4):6718-6728. doi: 10.1002/jcb.27969. Epub 2018 Dec 28. J Cell Biochem. 2019. PMID: 30592318
-
Nobiletin ameliorates monosodium urate-induced gouty arthritis in mice by enhancing AMPK/mTOR-mediated autophagy to inhibit NF-κB/NLRP3 inflammasome activation.Immunol Lett. 2025 Aug;274:106982. doi: 10.1016/j.imlet.2025.106982. Epub 2025 Feb 16. Immunol Lett. 2025. PMID: 39965668
-
Doliroside A attenuates monosodium urate crystals-induced inflammation by targeting NLRP3 inflammasome.Eur J Pharmacol. 2014 Oct 5;740:321-8. doi: 10.1016/j.ejphar.2014.07.023. Epub 2014 Jul 24. Eur J Pharmacol. 2014. PMID: 25064339
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Mechanism of flavonoids in the treatment of gouty arthritis (Review).Mol Med Rep. 2024 Aug;30(2):132. doi: 10.3892/mmr.2024.13256. Epub 2024 May 31. Mol Med Rep. 2024. PMID: 38818832 Free PMC article. Review.
Cited by
-
The dysregulation of immune cells induced by uric acid: mechanisms of inflammation associated with hyperuricemia and its complications.Front Immunol. 2023 Nov 20;14:1282890. doi: 10.3389/fimmu.2023.1282890. eCollection 2023. Front Immunol. 2023. PMID: 38053999 Free PMC article. Review.
-
Virtual screening combined with experimental verification reveals the potential mechanism of Fuzitang decoction against Gouty Arthritis.Heliyon. 2023 Nov 25;9(12):e22650. doi: 10.1016/j.heliyon.2023.e22650. eCollection 2023 Dec. Heliyon. 2023. PMID: 38058447 Free PMC article.
-
Current advances on the phytochemistry, pharmacology, quality control and applications of Anoectochilus roxburghii.Front Pharmacol. 2025 Jan 3;15:1527341. doi: 10.3389/fphar.2024.1527341. eCollection 2024. Front Pharmacol. 2025. PMID: 39830330 Free PMC article. Review.
-
Cynarin suppresses gouty arthritis induced by monosodium urate crystals.Bioengineered. 2022 May;13(5):11782-11793. doi: 10.1080/21655979.2022.2072055. Bioengineered. 2022. PMID: 35546047 Free PMC article.
-
Oral Bioavailability of Kinsenoside in Beagle Dogs Measured by LC-MS/MS: Improvement of Ex Vivo Stability of a Lactone-Containing Compound.Pharmaceutics. 2018 Jul 9;10(3):87. doi: 10.3390/pharmaceutics10030087. Pharmaceutics. 2018. PMID: 29987203 Free PMC article.
References
-
- Sabina EP, Rasool M, Mathew L, Ezilrani P, Indu H. 6-Shogaol inhibits monosodium urate crystal-induced inflammation – an in vivo and in vitro study. Food Chem Toxicol 2010; 48: 229–235. - PubMed
-
- Kienhorst LB, van Lochem E, Kievit W, Dalbeth N, Merriman ME, Phipps-Green A et al. Gout is a chronic inflammatory disease in which high levels of interleukin-8 (CXCL8), myeloid-related protein 8/myeloid-related protein 14 complex, and an altered proteome are associated with diabetes mellitus and cardiovascular disease. Arthritis Rheumatol (Hoboken, NJ) 2015; 67: 3303–3313. - PubMed
-
- Shi L, Xu L, Yang Y, Song H, Pan H, Yin L. Suppressive effect of modified Simiaowan on experimental gouty arthritis: an in vivo and in vitro study. J Ethnopharmacol 2013; 150: 1038–1044. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

